


Preview text:
CHE C M HE I M ST I R ST Y R Y LABO LAB R O AT R O AT R O Y R REPORT Experimen Experime t n t 3 : 3 : REDOX R TITR TI AT TR I AT O I N To T : Instructor: : Ph D Ph D H o H a o n a g n g L e L e So n So From: : D o T r T u r o u n o g n g A n A h n h T h T u h u - St S u t d u e d n e t n t ID: I BTBCIU BCI 1 U 4 1 0 4 4 0 6 4 Su S b u j b e j c e t c : t G e G n e e n r e a r l a l Che Ch m e i m s i t s r t y r y L a L b a o b r o a r t a o t r o y r Date: O c O t c o t b o e b r e r 1 2th t , ,2 0 2 1 0 5 1 I. INTRODUCTION Th T i h s i s e x e p x e p r e i r m i e m n e t n t s h s o h w o s w s u s u s t h t e h e t e t c e h c n h i n q i u q e u e t o o d e d t e e t r e m r i m n i e n e t h t e h e u n u k n n k o n w o n w n s o s l o u l t u i t o i n o n b y b y t h t e h e k n k o n w o n w so s l o u l t u i t o i n o n o f o f t h t e h e o x o i x d i a d t a i t o i n o - n r - e r d e u d c u t c i t o i n o n r e r a e c a t c i t o i n o n - - T I T T I RA T T RA I T O I N O . N . T h T e h e m a m i a n i l n y l y t i t tr t a r n a t n t u s u ed e d i n i th t i h s i s e x e p x e p r e i r m i e m n e t n t i s i p o p t o a t s a s s iu i m u m m a m n a g n a g n a a n t a e t e w i w t i h t h N = N 0 = . 0 0 . 5 0 5 t o t o t i t t i ra r t a e t e a g a a g i a n i s n t s t a a s ta t n a d n a d r a d d s ol o u l t u i t o i n o of o f H 2O2O4. 4 Th T e h n e n t h t e h e s tanda d r a d r i d z i e z d e KMn K O Mn 4s o s l o u l t u i t o i n o n w i w l i l l be b e u sed t o t o d eter e m r i m n i e n e t he co c n o c n e c n e t n r t a r t a i t o i n o n o f o f u n u k n n k o n w o n w n o x o a x l a i l c i c a c a i c d i d a n a d n d u n u k n n k o n w o n w n Fe 3+s o s l o u l t u i t o i n o . n Furthermore, , t he ex e p x e p r e im i e m n e t n t p r p o r v o i v d i e d s e s t h t e h e o v o e v r e v r i v e i w e w c o c n o c n e c p e t p t a b a o b u o t u t t h t e h e n a n t a u t r u e r e o f o f r e r d e o d x o x r e r a e c a t c i t o i n o n a n a d n d t h t e h re r l e a l t a i t o i n o s n h s i h p i p b e b t e w t e w e e n e n t h t e h e G E G W E W ( g ( r g a r m a m e q e u q i u va v l a e l n e t n w e w i e g i h g t h ) t , ) , t he h e v o v l o u l m u e m e a n a d n d with the h normality a mong t he o xidizing/reduc u ing a gents II. PR OCEDURE A. A Inst In r st u r m u ents: t On O e n e 5 0 5 0 m L m L b u b r u e r t e On O e n e 2 5 2 0 5 0 m L m L v o v l o u l m u e m t e r t i r c i c f l f a l s a k s On O e n e 1 0 1 0 0 0 m L m L g r g a r d a u d a u t a e t d e d c y c l y i l n i d n e d r e Th T r h e r e e e 2 5 2 0 5 0 m L m L b e b a e k a e k r e s r On O e n e 1 0 1 0 m L m L v o v l o u l m u e m t e r t i r c i c p i p p i e p t e Th T e h r e e r e 2 5 2 0 5 0 m L m L E r E l r e l n e m n e m y e e y r e r f l f a l s a k s s k On O e n e g l g a l s a s s s w a w t a c t h c On O e n e f u f n u n n e n l e l ( s ( m s a m l a l l l s iz i e z ) e On O e n e s t s ir i r r i r n i g n g r o r d o On O e n e m e m d e i d c i i c n i e n e d r d o r p o p p e p r e Wa W t a e t r e r b a b t a h t B. B. Exp Ex e p r e i r m i e m n e t n a t l a l Pro Pr c o e c d e u d r u e r : e 1. 1 Pre Pr p e a p r a e r e KMn O KMn 4s o s l o u l t u i t o i n o : n c a c lculate the e we w i e g i h g t h t o f o f KMn O KMn 4r e r q e u q i u r i e r d e d t o prepa p r a e e o f o f a a 0 . 0 05 5 N KMnO4s o s l o u l t u i t o i n o . n Af ter wei we g i h g i h ng g t he r e r q e u q i u red a mount KMn O KMn 4, , t r t a r n a s n f s e f r e r i t i t t o t o a a 2 5 2 0 5 0 m L m beak a er with 250
5 mL of distilled water. Mix the e solution t horough g ly by y vigorous swirling. The Th n e n t r t a r n a s n f s e f r e r i t i t t o t o a a d a d r a k r k b r b o r wn o wn b o b t o t t l t e l , e , d is i card u n u dissol o v l e v d e d s o s l o i l d. 2. 2 Cle Cl a e n a n t he b u b r u e r t e t wi with d i d s i t s illed e d w a water a n a d n t h t e h n e n r inse it three t im i e m s e s wi t wi h t h 5 5 m L mL po p r o t r ions p r p e r p e a p r a e r d e d KMn O KMn 4s o s l o u l t u i t o i n o , n a l a l l o l wi o n wi g n g t he r i r nse s olution t o drain t hough the t ip o f the h e buret ea e c a h c h t i t m i e m . e . Di s Di c s a c r a d r d t h t e h e r i r n i s n e s e s o s l o u l t u i t o i n o . n . F i F l i l l l t h t e h e b u b r u e r t e t wi t wi h t h KMn O KMn 4s o s l o u l t u i t o i n o n a n a d n d a l a l l o l w o i w t t o t dr d a r i a n i n t ough g h t he h e b u b r u e r t e t t i t p i p u n u t n i t l i l n o n o ai a r i r b u b b u bl b es rema m i a n i n i n t h t e h e t i t p. Re c Re o c r o d r d th t e h e b uret r e r a e d a i d n i g n b e b f e o f r o e r e b e b g e i g n i n n i n n i g n g t h t e h e t i t t i r t a r t a i t o i n o . n 3. 3 Sta St n a d n a d r a dizat a i t o i n o n o f o f p r p e r p e a p red d KMn O KMn 4solut u i t o i n o : n p i p pe p t e t s ep e a p r a a r t a e t e 1 0 1 0 m L m o L f o f s t s a t n a d n a d r a d r d o x o a x l a i l c i ac a i c d i d s o s l o u l t u i t o i n o n i n i t n o t o t h t r h e r e e e 2 5 2 0 5 0 m L m Er L l Er en e m n e m y e e y r e r f l f a l s a k s s k . s . Ad d Ad d ap a p p r p oxi x m i a m t a e t l e y l y 4 0 4 mL o L f distilled wat wa e t r e r t o t o e a e c a h c h f l f a l s a k s . k . I n I n t h t e h e f u f m u e m e h o h o o d o , d , c a c u a t u i t o i u o s u l s y l y a d a d d d 2 0 2 0 m L m o L f o f 6 6 N H N 2SO4s o s l o u l t u i t o i n o n t o t – – 0 ea e c a h c h f l f a l s a k s . k . W a W r a m r m t h t e h e f l f a l s a k s s k s i n i n t h t e h e wa t wa e t r e r b a b t a h t h t o t o 85 8 9 0 9 a n a d n d t i t tr t a r t a e t e t h t e h e h o h t o t s ol o u l t u i t o i n o s n ag a ainst the KMn O4s o s l o u l t u i t o i n o . n 4. 4 Det De e t r e m r i m n i a n t a i t o i n o n o f o f u n u k n no n wn o wn c on o c n e c n e t n r t a r t a i t o i n o n H 2C2O4s o s l o u l t u i t o i n o : n p i p pe p t e t s ep e a p r a a r t a e e 1 0 1 0 m L m o L f o unkno n wn conce
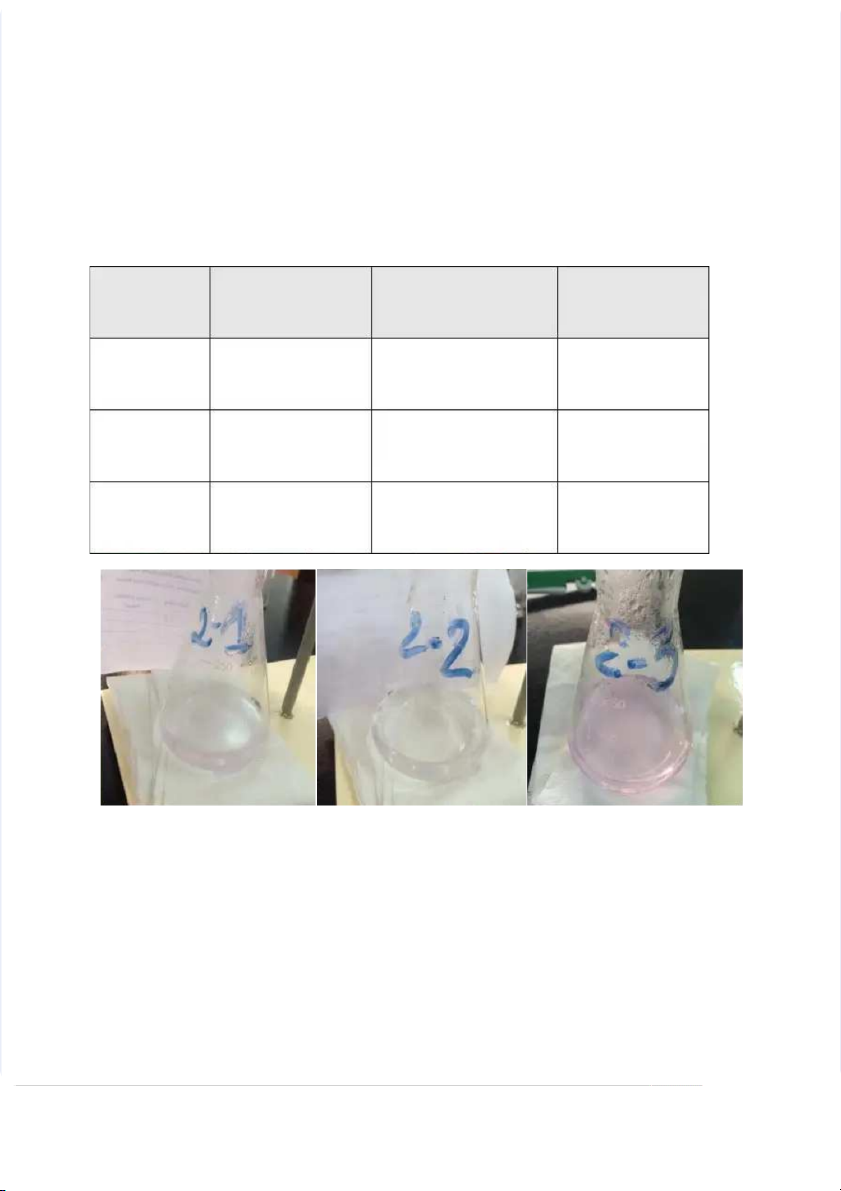
c ntration solution of H2C2O4 in i t n o t o t hr h e r e e e 2 5 2 0 5 0 m L m L Erl Er e l n e m n e m y e e y r e f l f a l s a k s s k s a n a d n p r p o r c o e c e e d e d a s a s d i d r i e r c e t c e t d e d i n i n t h t e h e s t s a t n a d n a d r a d r i d z i a z t a i t o i n o n p r p o r c o e c s e s s . s . Af t Af e t r e r f i f n i i n s i hi h n i g n g t h t e h e t i t t i r t a r tion, , c a c l a cul u a l t a e t th t e h e n o n r o ma m l a i l t i y t y o f o f t h t e h e u n u k n n k o n wn o wn c o c n o c n e c n e t n r t a r t a i t o i n o n H 2C2O4s o s l o u l t u i t o i n o ; n d e d termine the e a verage g e a n a d n th t e h e s t s a t n a d n a d r a d d d e d v e i v a i t a i t o i n o . n III I . . DATA T AND DIS I CUS U SION O 1. . TITRA TR TION ON OF KMn KM O4S O S LU OLUTION TI W I W T I H S TH TA T N A D N A D RD R D H 2C2O4S OL SO UTI LU O TI N ON No r No m r a m l a i l t i y t y o f o f t h t e h e s t s a t n a d n a d r a d r d H 2C2O4s o s l o u l t u i t o i n o , n NH2 H C 2 2 C O4 O = 0 = . 0 0 . 5 0 5 N Vol Vo u l m u e m e o f o f t h t e h e s t s a t n a d n a d r a d r d H 2C2O4 so s l o u l t u i t o i n o n u sed, VH2C2O4= 1 = 0 m L m Tria i l # # Bure Bur t e t t e t e re r a e d a i d n i g n g (mL) L Volu l me of o f KMn KM O4(mL) m L) Norm r alit i y of o KMn KM O4(N) 1 1 0. 0 0 . 0 0 - 0 1 - 0 1 . 0 0 . 0 0 0 10 1 . 0 0 . 0 0 0 0. 0 0 . 5 0 2 2 10 1 . 0 0 . 0 0 - 0 1 - 9 1 . 9 8 . 0 8 0 9.80 8 0 0. 0 0 . 5 0 1 5 3 3 19 1 . 9 8 . 0 8 - 0 2 - 9 2 . 9 6 . 0 6 0 9.80 8 0 0. 0 0 . 5 0 1 5 Da D t a a t a Ca C l a c l u c l u a l t a i t o i n: Normality of the e solution is cal a culated b y the e relationship VKMnO4x x N KMnO4= = V H2C2O4 H2C2O4x N H2C2O H2C 4 2O The Th e No r No m r a m l a i l t i y t y o f KMn O KMn 4 is: : NKMnO4 = ( = V ( H2 H C 2 2 C O 2 4x x N H2 H C 2 2 C O 2 4)/VKMn M O n 4 O For Fo r t h t e h e Tr i Tr al a l 1 : 1 : N KMn K O Mn 4 = 0 = . 0 0 . 5 0 x 5 10/10 1 0 = 0 = . 0 05 For Fo r t h t e h e Tr i Tr al a l 2 : 2 : N KMn K O Mn 4 = 0.05x 5 1 x 0 1 /9. 9 8 . 8 = 0. 0 0 . 5 0 1 5 For Fo r t h t e h e Tr i Tr al a l 3 : 3 : N KMn K O Mn 4 = 0.05x 5 1 x 0 1 /9. 9 8 . 8 = 0. 0 0 . 5 0 1 5 Aver e age e Norma m lity t of KMn KM O4 = (0 ( . 0 0 . 5 0 5 + 0.05 0 1 5 1 + + 0 . 0 0 . 5 0 1 5 )/3 3 = 0 = . 0 0 . 5 0 0 5 7 0 7 ( N) 2. . TITRA TR TION ON OF UNKN N OWN OW CONC ON EN E TRA TR TION ON H2C2O4 SOLU SOLUTION TION WI W TH I STAN TA DARD D KMn KM O4S O S LUTI OLU O TI N ON No r No m r a m l a i l t i y t y o f o f t h t e h e s t s a t n a d n a d r a d r d KMn O KMn 4 so s l o u l t u i t o i n o , n N(KMn ( O KMn 4) = 0.05 0 5 N Vol Vo u l m u e m e o f o f t h t e h e u n u k n n k o n wn o wn H 2C2O4 so s l o u l t u i t o i n o n u s u e s d e , d V(H2C2O4) = 1 = 0 mL Tria i l # # Bure Bur t e t t e t e re r a e d a i d n i g n g (mL) L Volu l me of o f KMn KM O4 Norm r alit i y of (m ( L m ) L H2C2O4( N ( ) N 1 1 0. 0 0 . 0 0 - 0 7 - . 7 9 . 0 9 0 7.90 0 0. 0 0 . 3 0 9 3 5 9 2 2 10 1 . 0 0 . 0 0 - 0 1 - 8 1 . 8 1 . 0 1 0 8.10 0 0. 0 0 . 4 0 0 4 5 0 3 3 10 1 . 0 0 . 0 0 - 0 1 - 7 1 . 7 8 . 0 8 0 7.80 0 0.039 3 Da D t a a t a Ca C l a c l u c l u a l t a i t o i n: Normality of the e solution is cal a culated b y the e relationship VKMnO4x x N KMnO4= = V H2C2O4 H2C2O4x N H2C2O H2C 4 2O The Th e No r No m r a m l a i l t i y t y o f KMn O KMn 4 is: : NH2C2 C O 2 4 O = ( = V ( KMnO4x NKMnO4)/VH2C2O 2 4 O For Fo r t h t e h e Tr i Tr al a l 1 : 1 : N H2 H C 2 2 C O4 = 0.05x 5 7 x . 7 9/10 = 0. 0 0 . 3 0 9 3 5 For Fo r t h t e h e Tr i Tr al a l 2 : 2 : N H2 H C 2 2 C O4 = 0 = . 0 0 . 5 0 x 5 8.1/10 = 0 = . 0 0405 For Fo r t h t e h e Tr i Tr al a l 3 : 3 : N H2 H C 2 2 C O4 = 0 = . 0 0 . 5 0 x 5 7. 7 8 . / 8 10 = 0 = . 0 03 0 9 3 Aver e age e Norma m lity t of KMn KM O4 = (0 ( . 0 0 . 3 0 9 3 5 9 +0. +0 0 . 4 0 05 0 +0 5 . +0 0 . 3 0 9 3 )/3 = = 0. 0 0 . 3 0 9 3 7 9 (N) 3. . TITR TI ATI A ON ON OF O UNKN N OWN OW CON O CENTR T ATION TI Fe SO S 4S OL SO UTI LUTION ON W I W TH TH STAN TA DARD D KMn KM O4S O S LUTI OLU O TI N ON No r No m r a m l a i l t i y t y o f o f t h t e h e s t s a t n a d n a d r a d r d KMn O KMn 4 so s l o u l t u i t o i n o , n N(KMn ( O KMn 4) ) = 0 = . 0 0 . 5 0 5 N Vol Vo u l m u e m e o f o f t h t e h e u n u k n n k o n wn o wn H 2C2O4 so s l o u l t u i t o i n o n u s u e s d e , d V(Fe ( SO Fe 4) = 10 0 m L m Tria i l # # Bure Bur t e t t e t e re r a e d a i d n i g n g (mL) L Volu l me of o f KMn KM O4 (mL) L) No N r o m r a m l a i l t i y of FeSO S 4(N ( ) N 1 1 10 1 . 0 0 . 0 0 - 0 1 - 5 1 . 5 8 . 0 8 0 5.80 0 0.0 . 2 0 9 2 2 2 10 1 . 0 0 . 0 0 - 0 1 - 5 1 . 5 9 . 0 9 0 5.90 0 0.0295 9 3 3 10 1 . 0 0 . 0 0 - 0 1 - 6 1 . 6 0 . 0 0 0 6.00 0 0. 0 0 . 3 0 Da D t a a t a Ca C l a c l u c l u a l t a i t o i n: Normality of the e solution is cal a culated b y the e relationship VKMnO4x x N KMnO4= = V FeSO4 FeSO4x x N FeSO4 FeSO4 The Th e No r No m r a m l a i l t i y t y o f KMn O KMn 4 is: : NFeSO4 = ( = V ( KMn MnO4x x N KMnO4 )/VFeSO4 For Fo r t h t e h e Tr i Tr al a l 1 : 1 : N FeS F O eS 4 O = 0 = . 0 0 . 5 0 x 5 5. 5 8 . / 8 10 1 0 = 0.029 2 For Fo r t h t e h e Tr i Tr al a l 2 : 2 : N FeS F O eS 4 O = 0 = . 0 0 . 5 0 x5. 5 9 . / 9 1 / 0 1 0 = 0.029 2 5 9 For Fo r t h t e h e Tr i Tr al a l 3 : 3 : N FeS F O eS 4 O = 0 = . 0 0 . 5 0 x 5 6 x / 6 10 1 0 = 0 = . 0 0 . 3 0 Aver e a r ge e N o N r o m r a m l a i l t i y t y o f o f Fe S Fe O S 4 = (0 ( . 0 0 . 2 0 9 2 +0 9 . +0 0 . 2 0 9 2 5 9 +0 5 . +0 0 . 3 0 ) 3 / ) 3 / 3 = = 0 . 0 0 . 2 0 9 2 5 9 5 ( N) ( IV I . V . C ON C C ON L C US LU I S O I N ON Wi W t i h t h t h t e h e m e m t e h t o h d o d T i T t i r t a r t a i t o i n o n i n i n t h t i h s i s e x e p x e p r e im i e m n e t n , t , we we c a c n a n c a c l a c l u c l u at a e t e t h t e h e u n u k n n k o n wn o wn c o c n o c n e c n e t n r t a r t a i t o i n o so s l o u l t u i t o i n o n b y b y a d a d d i d ng n g t h t e h e k n k o n wn o wn v o v l o u l m u e m e o f o f t h t e h e s t s a t n a d n a d r a d r i d z i e z d e d s o s l o u l t u i t o i n o n u n u t n il i l t h t e h e r e r a e c a t c i t o i n o n b e b t e we t e we n e th t e h m e m r ea e c a h c e h s e s n e n u e t u r t a r l a i l z i a z t a i t o i n o n t h t r h o r u o g u h g h t h t e h e r e r l e a l t a i t o i n o s n h s i h p i p V ox o idx Noxid i = Vred r x x N red. At the h e e nd n d o f ti t t i r t a r t a i t o i n o , n , t h t r h e r e e e o f o f f o f u o r u r v a v r a i r a i b a l b e l s e s wi l wi l l l b e b e k n k o n wn o wn a n a d n d t he h e u n u k n n k o n wn o wn v a v r a i r ab a l b e l e c a c n a n b e b e d e d t e e t r e m r i m n i e n d e . d