


Preview text:
Phạm Minh Thành – IELSIU22294 I/ PURPOSES - General purposes
- To examine the effects of concentration, temperature, and catalysts on reaction rates. - Applications
Reactant concentration. Increasing the concentration of one or more reactants will often increase the
rate of reaction. This occurs because a higher concentration of a reactant will lead to more collisions of
that reactant in a specific time period.
Physical state of the reactants and surface area. If reactant molecules exist in different phases, as in a
heterogeneous mixture, the rate of reaction will be limited by the surface area of the phases that are in
contact. For example, if a solid metal reactant and gas reactant are mixed, only the molecules present on
the surface of the metal are able to collide with the gas molecules. Therefore, increasing the surface area
of the metal by pounding it flat or cutting it into many pieces will increase its reaction rate.
Temperature. An increase in temperature typically increases the rate of reaction. An increase in
temperature will raise the average kinetic energy of the reactant molecules. Therefore, a greater
proportion of molecules will have the minimum energy necessary for an effective collision (Figure. 17.5
“Temperature and Reaction Rate”).
Presence of a catalyst. A catalyst is a substance that accelerates a reaction by participating in it without
being consumed. Catalysts provide an alternate reaction pathway to obtain products. They are critical to
many biochemical reactions. They will be examined further in the section “Catalysis.” II/ METHODS
Equipment: Twelve test tubes, one test tube rack, one cylinder, three 80mL beakers, three 200mL
beakers, one volumetric pipette, one stirring rod, one spoon, 50 C water bath, o 90 C water bath o Methods:
1. Effect of time on the reaction rate
Reaction time is influenced by concentration, as seen in the first experiment. The first beaker (200 mL)
was prepared and filled with 90 mL of 0.1M (NH4)2S2O in order to conduct this experiment. 8
Subsequently, 60mL of 0.005M Na2S2O3 was added to the second beaker (80mL) and 90mL of 0.2M KI
was added to the third beaker (200mL).
Because we were out of test tubes, we split the experiment into two sections. Tubes A1 to A6 and B1 to
B6 were included in Part 1; tubes A7 to A11 and B7 to B11 were included in Part 2.
In section 1, a measuring cylinder was used to add 10 mL (NH4)2S2O to the tubes, which included tubes 8
A1 through A6. Next, using a volumetric pipette, 5 mL of Na2S2O and 4 mL of starch were added, 3
respectively, to the tubes numbered A1 through A6. Next, we continue to fill the tubes from B1 to B6
with 10mL of KI, 8.5mL of KI and 1.5mL of distilled water, 7mL of KI and 3mL of distilled water,
5.5mL of KI and 4.5mL of distilled water, 4mL of KI and 6mL of distilled water, and 2.5mL of KI and
7.5mL of distilled water using a volumetric pipette.
Combine solutions A and B, then transfer tube B1 into tube A1. After that, a stirring rod was used to
agitate the chemical, and the time was noted. The moment deep blue color appeared for the first time,
time paused. The outcome was noted and entered into a datasheet. Up to the point where tubes A6 and B6
were combined, the trials were repeated. In part 2, 8.5mL (NH ) S O ) S O 4 2 2
8 and 1.5mL distilled water, 7mL (NH4 2 2
and 3mL distilled water, 5.5mL 8 (NH ) S
and 4.5mL distilled water, 4mL (NH ) S O ) S and 4 2 2
and 6mL distilled water, 2.5mL (NH 4 2 2O8 8 4 2 2O8
7.5mL distilled water were added respectively to the tubes form A7 to A11 by a volumetric pipette. Those
tubes were also filled with 5mL Na S O 2 2
and 4mL starch. The tubes from B7 to B11 were added 10mL K 3 I.
Then mix solution A and solution B, and repeat the steps as in part 1
Finally, the data of the concentration of iodide and peroxydisulfate ion was plotted. In the first graph, the
concentration of iodide ion versus time for mixtures from 1 to 6 was plotted. In the second graph, the
concentration of peroxydisulfate ion versus time for mixture of 1, 7, 8, 9, 10 and 11.
2. Effect of temperature on the reaction rate
The second experiment served as an example of how temperature affects reaction pace. 5mL of 0.33M
H2C2O4 was put into tubes 1A, 2A, and 3A in the preparation section. Next, using a volumetric pipette,
add 1 mL of KMnO4 to tubes 1B, 2B, and 3B for testing.
After that, tubes 1A and 1B were put at room temperature and moved on to the procedure section. One
tube (1A) was filled with tube 1B. The moment the purple tint vanished was noted and entered into the
datasheet. For almost three minutes, tubes 2A and 2B were submerged in a water bath at 50 degrees
Celsius. Tube 2B was then filled into tube 2A after that. Note the time and write it in the datasheet when
the purple color vanished. For three minutes, Tubes 3A and 3B were submerged in a water bath set at
90°C. Pour tube 3B into tube 3A after that. noted and entered in the datasheet the moment the solution became colorless.
3. Effect of a catalyst on the reaction rate
The purpose of the second experiment was to demonstrate how a catalyst affects the rate of reaction. In
the preparation section, prepare seven test tubes and fill the 80 ml beaker with 40 mL of 3% H2O . 2
Each tube received 5 milliliters of 3% H2O during the process. Next, fill tubes 1 through 7 with MnCl 2 , 2 MnO2, NaCl, CaCl , Zn, KNO 2
3, and Fe(NO3) , in that order. shook the mixture and measured the reaction 3
rate, or how long it took for bubbles to form. In the end, the reaction rates were ordered from fastest to slowest in descending order.
III/ SAFETY PRECAUTIONS -
When you breathe in potassium nitrate (KNO ), it can have an effect on you. Contact 3 irritates the
eyes and skin, as well as the nose and throat, causing sneezing and coughing. High amounts can
impair the ability of the blood to transport oxygen, resulting in headaches, weariness, dizziness,
and a blue hue to the skin and lips (methemoglobinemia). Higher levels can result in difficulty
breathing, collapse, and even death. Potassium nitrate may harm the kidneys and induce anemia. -
Skin problems are among the possible side effects of sodium thiosulphate (Na2S2O ). This 3
substance has a low order of toxicity, therefore it may cause moderate skin irritation, ocular
irritation, mechanical eye irritation, upper respiratory tract irritation, mucous membrane irritation, and ingestion irritation. -
When inhaled, ammonium persulfate (NH4)2S2O . Can have an impact on you. Skin and eyes 8
might become irritated by contact. Exposure may cause throat and nasal irritation. Ammonium
persulfate inhalation can irritate the lungs. Increased exposures may result in pulmonary edema, a
medical emergency caused by an accumulation of fluid in the lungs. Allergies to ammonium
persulfate may occur on the skin. Although ammonium persulfate is not combustible, it is a potent
oxidizer that facilitates the burning of other materials. -
Inhaling ferric nitrate Fe(NO3)3 can have an effect on you. Contact with Ferric Nitrate can cause
skin and eye irritation, while inhaling it can cause coughing and wheezing. High amounts of this
molecule may impair the blood's ability to transport oxygen, resulting in headache, weariness,
dizziness, and a blue hue to the skin and lips (methemoglobinemia). Repeated high exposures may
cause excessive Iron buildup in the body, resulting in nausea, vomiting, stomach pain,
constipation, and black bowel movements. Ferric Nitrate may harm the liver. Although not
combustible, it is a powerful oxidizer that improves the combustion of other chemicals. - Wear your eye protection. - Do not inhale any fumes. -
Sulfur dioxide is toxic and corrosive. Dispose of the solution immediately after the experiment
following your teacher's instructions. - Wash your hands when finished
IV/ SUGGESTED QUESTIONS
1/ What is the objective of today lab work? -
To check the effect of concentration, temperature, and catalysts on reaction rates.
2/ What is the rate of a chemical reaction? -
The rate of a chemical reaction is the change in concentration over the change in time and is a
metric of the "speed" at which a chemical reactions occurs.
3/ How can the rate of a reaction be determined? -
There are two ways to determine the rate of a reaction: either measure the time it takes for one or
more of the reactants is used up, or for the products to be formed.
4/ What is the unit expression of reaction rate? -
Reaction rates are usually expressed as the concentration of reactant consumed or the
concentration of product formed per unit time. The units are thus moles per liter per unit time, written as M/s, M/min, or M/h.
5/ Please list out factors that can affect the rate of a reaction? + The nature of the reactants + Temperature of the reaction
+ Concentration of the reactants
+ The surface area of the reactants + The presence of a catalyst
+ The pressure the reaction is under.
6/ How does temperature affect the reaction rate? -
The temperature increases, the rate reaction increases and vice versa because the temperature
increases, the thermal movement speed of molecules increases, so the molecules colide more and
more strongly that leads to the reacion occurs faster.
7/ How does the concentration of reactants affect the reaction rate? -
The concentration of reactants increases, the rate of reaction increases because when the
concentration of suntances that precede the reaction increases, the collision between them
increases, so the reaction occurs faster.
8/ What is a catalyst? Is it consumed during the reaction? -
A catalyst is a substance that speeds up the rate of a chemical reaction but is not consumedduring the course of the reaction.
9/ In part 1, what is the role of starch? Please explain -
The starch is used to determining the existence of iodine excess after reactions. Because the
excess iodine will react with starch to form a deep blue solution.
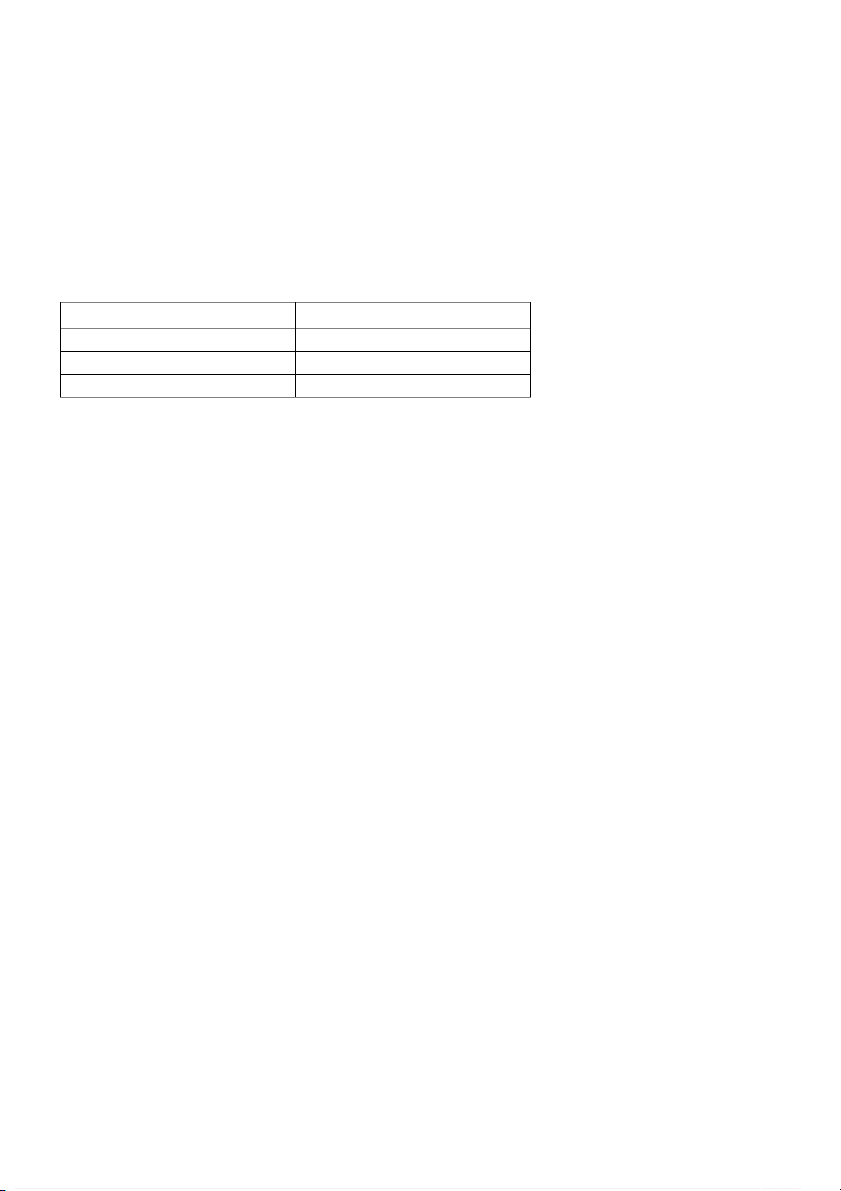
10/ In part 2, please predict the outcome of the experiment Description of condition Predicted outcome Room tempurature Slow 500C Faster than 900C Fastest




