




















Preview text:
Page 1
Chemical Engineering Laboratory EXPERIMENT REPORT
Experiment 1: Chemical reactions: Chemical Engineering Laboratory By Group 2 For Dr. Doan Hoai Linh Group 2
Nguyen Quoc Anh Khoa BEBEIU21068 Doan Bao Ngan BEBEIU21243
Nguyen Hoang Phi Long ITITIU18250
Ho Nguyen Minh Nguyen BEBEIU21247 Nguyen Thi Cam Ly IELSIU21323
I affirm that I have carefully proofread each report section, and that each satisfies all applicable
criteria listed on the report checklist Page 2
Chemical Engineering Laboratory Abstract:
Experimenting with a chemical reaction demonstrated the phenomena that emerged when
chemicals interacted with one another and transformed in a variety of, including combustion
reactions, acid-base reactions, precipitation, and the creation of new compounds. Observations
showed that new chemical compounds were produced as a balanced reaction, result of the
interactions, along with state changes, colour changes, smoke reactions, and temperature changes
of alcohol light while using certain chemicals. Finally, the results of reactions using potassium
permanganate (KMnO4) or hydrogen peroxide (H2O2) were oxidation-reduction processes. The
test on acid and base in reactions involving the ions Fe2+, Fe3+ and Al3+, Cu2+ made it abundantly
evident how precipitates and acid or base are related. In this experiment, fundamental sorts of
reactions that may be found in protocols were shown. Students' understanding of chemical
reactions' basic concepts and their various impacts on various substances allows them to avoid unneeded harm. Page 3
Chemical Engineering Laboratory Table of Contents Introduction 6 Theory 6 Experimental method 8 Result and discussion 12 Conclusion 23 References 25 Table of figures
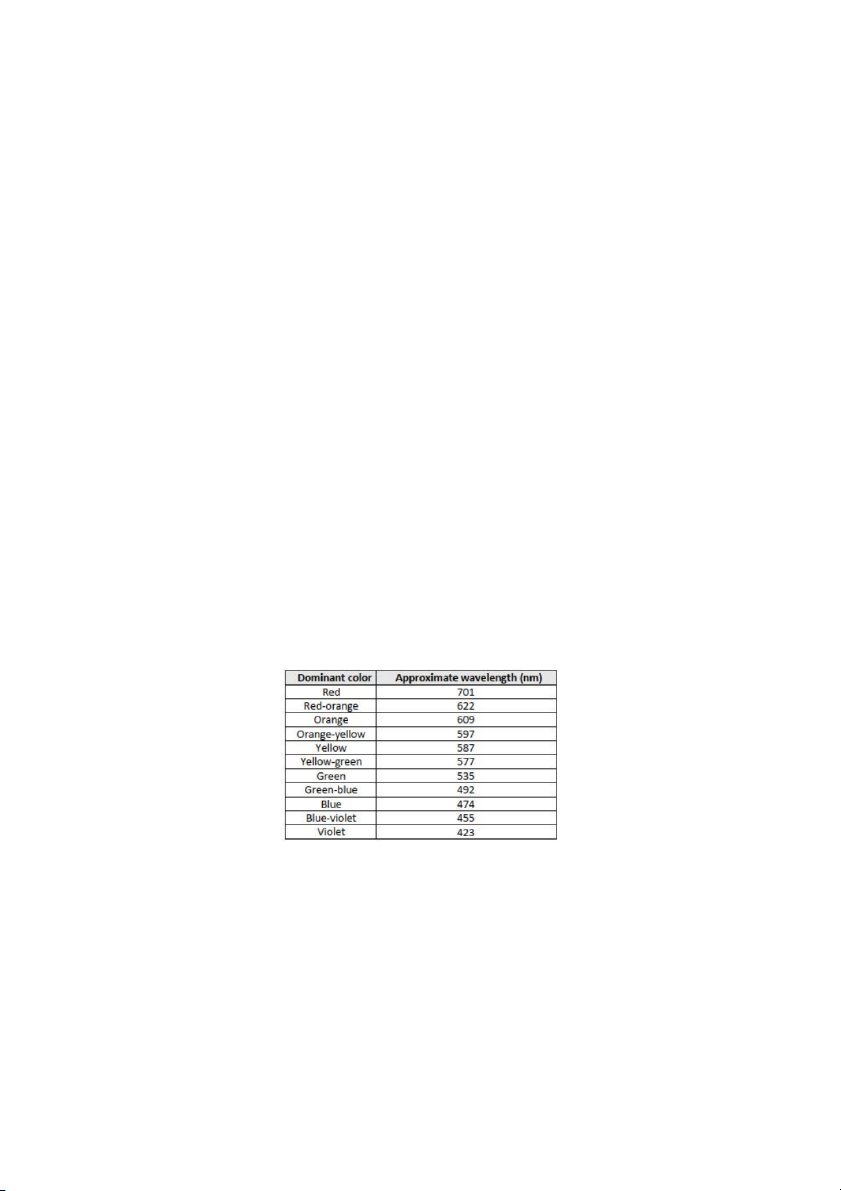
Figure 1. Wavelength data for indicated color's mid-range Figure 2. (a) Beaker Figure 2. (b) Teset tube Figure 2. (c) Test tube holder Figure 2. (d) Test tube rack Figure 2. (e) Dropper
Figure 3. (a) Reaction of copper sulfate (CuSO4) and sodium hydroxide (NaOH)
Figure 3. (b) Reaction of the mixture after adding more sodium hydroxide (NaOH)
Figure 3. (c) Reaction of copper sulfate (CuSO4) and sodium hydroxide (NaOH)
Figure 3. (d) Reaction of the mixture after adding ammonium hydroxide (NH4OH)
Figure 4. (a) Reaction of potassium chloride (KCl) and silver nitrate (AgNO3) Page 4
Chemical Engineering Laboratory
Figure 4. (b) Reaction of the mixture after adding ammonium hydroxide (NH4OH)
Figure 4. (c) Reaction of potassium bromide (KBr) and silver nitrate (AgNO3)
Figure 4. (d) Reaction of the mixture after added ammonium hydroxide (NH4OH)
Figure 5. (a) Reaction of hydrogen peroxide (H2O2) in sulfuric acid (H2SO4) with potassium permanganate (KMnO4)
Figure 5. (b) Reaction of hydrogen peroxide (H2O2) in sulfuric acid (H2SO4) with potassium iodide (KI)
Figure 5. (c) Reaction of hydrogen peroxide (H2O2) with manganese dioxide (MnO2)
Figure 6. (a) Reaction of potassium permanganate (KMnO4) in sulfuric acid (H2SO4) with sodium sulfite (Na2SO3)
Figure 6. (b) Reaction of potassium permanganate (KMnO4) in sodium hydroxide (NaOH)) with sodium sulfite (Na2SO3)
Figure 6. (c) Reaction of potassium permanganate (KMnO4) in water with sodium sulfite (Na2SO3)
Figure 7.A. (a) Reaction of iron trichloride (FeCl3) and potassium hydroxide (KOH)
Figure 7.A. (b) Reaction of iron trichloride (FeCl3) and ammonium hydroxide (NH4OH)
Figure 7.B. (a) Reaction of iron sulphate (FeSO4) and potassium hydroxide (KOH)
Figure 7.B. (b) Reaction of iron sulphate (FeSO4) and ammonium hydroxide (NH4OH)
Figure 8. (a) Reaction of aluminum sulphate Al2(SO4)3 and sodium hydroxide (NaOH) Page 5
Chemical Engineering Laboratory
Figure 8. (b) Reaction of mixture aluminum sulphate Al2(SO4)3 and sodium hydroxide (NaOH)
after added hydrochloric acid (HCl)
Figure 8. (c) Reaction of mixture aluminum sulphate Al2(SO4)3 and sodium hydroxide (NaOH)
after added more sodium hydroxide (NaOH)
Figure 9. (a) Red flame happened when burning lithium chloride (LiCl)
Figure 9. (b) Yellow flame happened when burning sodium chloride (NaCl)
Figure 9. (c) Purple flame happened when burning potassium chloride (KCl)
Figure 9. (d) Red orange flame happened when burning calcium dichloride (CaCl2)
Figure 9. (e) Orange flame happened when burning barium chloride (BaCl2) List of tables
Table 1. Table of reactions of copper ion
Table 2. Table of reactions of silver halides
Table 3. Table of reactions of hydrogen peroxide
Table 4. Table of reactions of potassium permanganate
Table 5. Table of reactions of ferric ion (Fe3+)
Table 6. Table of reactions of ferrous ion (Fe2+)
Table 7. Table of reactions of aluminum ion
Table 8. Table of flame testing Page 6
Chemical Engineering Laboratory Introduction
The study of chemical reactions has long been a focus of several laboratories. Chemical reactions
brought new products and materials to life with various features and configurations from each
equation put into practice. The precipitate, evaporates changed the color of the flame of the
alcohol lamp, and recognizes the fundamental types of chemical reactions: synthesis,
decomposition, oxidation-reduction, substitution, and exothermic. This experiment will teach
participants how to recognize these phenomena as well as the different concentrations and
volumes of common chemicals that react. Theory
Five types of reaction: synthesis, decomposition, single displacement, double displacement, and combustion.
Synthesis reaction: are processes that take place when two distinct atoms or molecules
combine to produce a new chemical or molecule. When a synthesis process takes place,
energy is often released, and the reaction is exothermic. An endothermic result, however, is
also conceivable. One of the main categories of chemical reactions is called synthesis, which
also includes single displacement, double displacement, and combustion processes.[1]
Decomposition: A chemical breaking down into two or more simpler compounds is known as
a decomposition process. Energy input in the form of heat, light, or electricity is necessary
for the majority of decomposition processes.[2]
Single displacement: One reactant is substituted with one ion of a different reactant in a
single-displacement reaction. The exchange or replacement of one cation with another cation
or one anion with another anion occurs in single-displacement reactions.[3] Page 7
Chemical Engineering Laboratory
Double displacement: In a double displacement reaction, two chemicals react and the
positive (cation) and negative (anion) ions of the two reactants swap positions, creating two new compounds or products.[4]
Combustion: When a chemical combines with oxygen gas, it produces a combustion process
that releases energy in the form of heat and light oxygen (O2) must be a reactant in combustion applications.[5]
The relationship between wavelength, frequency, and speed of an electromagnetic wave in flame test: C = λ x v
Where C is the speed of light (3 x 108 m/s) λ is the wavelength (nm) v is frequency
With wavelength data for the indicated color's mid-range are provided:
Figure 1. Wavelength data for indicated color's mid-range
The equation gives the energy per photon: Ephoton = h x v Page 8
Chemical Engineering Laboratory
Where Ephoton is the energy per photon (J)
h is Planck’s constant (6.626 x 10-34 J.s) v is frequency
Calculate the frequency, speed and Ephoton of the flame using the various reactants and the
combustion reaction of the various substances using that equation.
Application: This would be beneficial to apply to the day-to-day work of many professions in the
field of chemistry by practicing a few reactions. As a consequence, that becomes easier for
people to see how crucial chemistry is to modern life, and eventually and becomes practical to
carry out studies on chemicals. Experimental method 1. Techniques:
There were two main techniques used in this experiment: flame test and substance mixing to observe the phenomenon.
The flame test is a qualitative chemical test used to assist detect the identification or potential
identity of a metal or metalloid ion contained in an ionic compound. When the compound is
exposed to the flame of a gas burner, it may emit a distinct hue that is apparent to the human eye gh 2. Materials
Chemicals: copper sulfate (CuSO4), sodium hydroxide (NaOH), ammonium hydroxide (NH4OH),
potassium chloride (KCl), silver nitrate (AgNO3), potassium bromide (KBr), hydrogen peroxide Page 9
Chemical Engineering Laboratory
(H2O2), potassium permanganate (KMnO4), potassium iodide (KI), sulfuric acid (H2SO4),
manganese dioxide (MnO2), sodium sulfide (NaSO3), distilled water, ferric chloride (FeCl3),
potassium hydroxide (KOH), iron (II) sulfate (FeSO4), aluminum sulfate (Al2(SO4)3), hydrochloric acid (HCl).
Equipment: test tube, dropper, test tube rack, test tube holder, beaker. Figure 2. (a) Beaker Figure 2. (b) Test tube Figure 2. (c) Test tube holder Figure 2. (d) Test tube rack Figure 2.(e) Dropper 3. Procedure Reactions of copper ion (Cu2+)
Prepared two test tubes, one labelled 1 and the other marked 2. Added 10 drops of 0.5M copper
sulfate (CuSO4) into two tubes, then added 10 drops of 2M sodium hydroxide (NaOH) into tube Page 10
Chemical Engineering Laboratory
1 and 10 drops of 2M ammonium hydroxide (NH4OH). Gently combined the chemicals in two
test tubes and watched the results. After that, in tube 1, added 10 drops of 2M sodium hydroxide
(NaOH) solution and 10 drops of 2M ammonium hydroxide (NH4OH) solution for tube 2.
Continue to mix the liquids in the two tubes and examine any potential phenomena. Reactions of silver halides
Section 1: Reactions of Potassium Chloride (KCl)
To two pre-prepared test tubes, added 10 drops of 0.5M potassium chloride (KCl). Added 10
drops of 0.1M silver nitrate (AgNO3) to the first tube, gently mixed the contents, and left for
around 2 minutes to examine the results. The second test tube contained 10 drops of 0.1M silver
nitrate (AgNO3) and 10 drops of 2M ammonium hydroxide (NH4OH), respectively; mixed the
materials in the tube and waited for 2 minutes to observe.
Section 2: Reactions of Potassium Bromide (KBr)
Added 10 drops of 0.5M potassium bromide (KBr) to two pre-prepared test tubes. To the first
tube, added 10 drops of 0.1M silver nitrate (AgNO3), gently mixed the contents, and allowed for
around 2 minutes to evaluate the findings. The second test tube included 10 drops of 0.1M silver
nitrate (AgNO3) and 10 drops of 2M ammonium hydroxide (NH4OH); mixed the contents for 2
minutes in the tube then observed.
Reactions of hydrogen peroxide (H2O2)
Prepared three test tubes, labelled 1, 2, and 3, and placed one drop of 0.1M potassium
permanganate (KMnO4) in the first tube, 5 drops of 0.1M potassium iodide (KI) in the second,
and 10 drops of 3% hydrogen peroxide (H2O2) in the third. Continued adding 5 drops of 2M Page 11
Chemical Engineering Laboratory
sulfuric acid (H2SO4) and 5 drops of 3% hydrogen peroxide (H2O2) to tubes 1 and 2. Put a pinch
of manganese dioxide (MnO2) in tube 3. Finally, carefully mixed the contents inside the three
tubes and wait for 2 minutes to watch the occurrence.
Reactions of potassium permanganate (KMnO4)
Prepared three test tubes, each containing ten drops of 0.5M sodium sulfide (NaSO3), and
labelled them 1,2,3. In turn, added 5 drops of 2M sulfuric acid (H2SO4), 5 drops of 6M sodium
hydroxide (NaOH), and 5 drops of distilled water to tubes 1, 2, and 3. Finally, added 5 drops of
0.1M potassium permanganate (KMnO4) to each of the three test tubes, mixed thoroughly, and
evaluated the findings of those three test tubes.
Reactions of ferric ion (Fe3+) and ferrous ion (Fe2+) Section 1: Ferric ion (Fe3+)
Prepared two test tubes, each with 10 drops of 0.5M ferric chloride (FeCl3). Filled the first tube
with 5 drops of 2M potassium hydroxide (KOH) and the second tube with 5 drops of 2M
ammonium hydroxide (NH4OH). Examined the outcomes of the two tubes after the solution inside was blended. Section 2: Ferrous ion (Fe2+)
Filled two test tubes with 10 drops of 0.5M iron (II) sulfate (FeSO4). Inserted the first tube with 5
drops of 2M potassium hydroxide (KOH), then added five drops of 2M ammonium hydroxide
(NH4OH) into the second tube. Examined the results of the two tubes after the solution within has been mixed.
Reactions of aluminum cation (Al3+) Page 12
Chemical Engineering Laboratory
Prepared two test tubes, each with ten drops of 0.5M aluminum sulfate (Al2(SO4)3). Observed the
effect after adding 5 drops of 2M sodium hydroxide (NaOH) to each tube. Continue to add 20
drops of 2M hydrochloric acid (HCl) in tube 1 and 20 drops of 2M sodium hydroxide (NaOH) in
tube 2 after the initial observation. Mixed the solutions well in both tubes and repeated the observation. Flame test
While washing the loop with distilled water, light the flame of the alcohol lamp. Once the loop
has been cleaned, immersed it in the sample solution lithium chloride, sodium chloride,
potassium chloride, calcium chloride and barium chloride (LiCl, NaCl, KCl, CaCl₂ and BaCl₂),
to get the required sample. To acquire the results, insert the sampled loop into the frame and
examine the color of the flame. Clean up the loop in preparation for the next trial. The procedure
is the same with the other reagents. Result and discussion
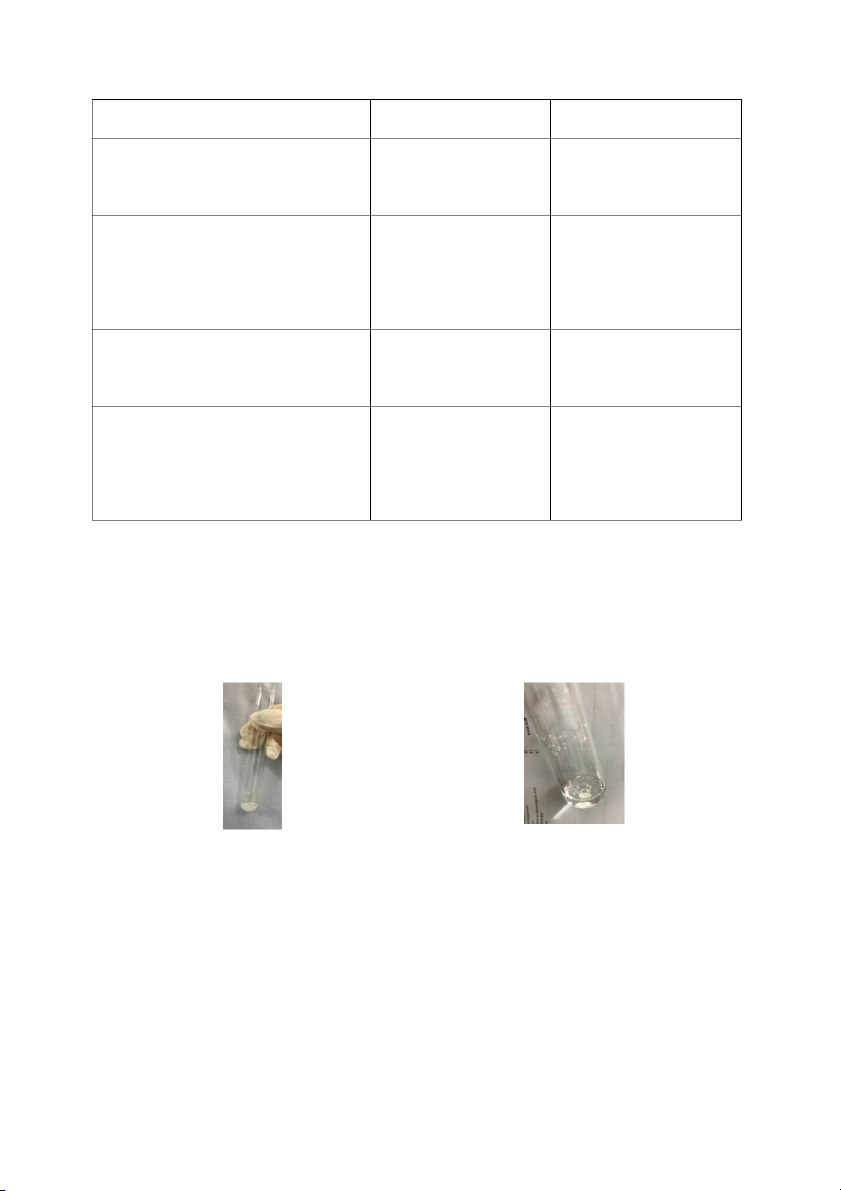
1. Reactions of copper ion (Cu2+)
Table 1. Table of reaction of copper ion Reaction Observation Chemical Equation 0,5M copper sulfate (CuSO4) Blue precipitate CuSO4 + 2NaOH → + 2M sodium hydroxide (NaOH) appeared Cu(OH)2 ↓+ Na2SO4 0.5M copper sulfate (CuSO4) Blue precipitate CuSO4 + H4OH →
+ 2M ammonium hydroxide (NH4OH) appeared, then the Cu(OH)2 ↓+ (NH4)2SO4 precipitate dissolved The reaction continued Page 13
Chemical Engineering Laboratory to form a blue Cu(OH)2 + (NH4)2SO4 → solution 4H2O + [Cu(NH3)4](OH)2
The experiment matched theoretical data because the precipitation was copper (II) hydroxide
(Cu(OH)2), sodium hydroxide (NaOH) made lighter blue color (Figure 1.(a), Figure 1.(c)) but by
adding more sodium hydroxide (NaOH) (Figure 1. (b)) and ammonium hydroxide (NH4OH)
(Figure 1. (d)) it completely dissolved the precipitation and left with clear solution.
Figure 3.(a) Reaction of copper sulfate
Figure 3.(b) Reaction of the mixture after
(CuSO4) and sodium hydroxide (NaOH)
adding more sodium hydroxide (NaOH)
Figure 3.(c) Reaction of copper sulfate
Figure 3.(d) Reaction of the mixture after
(CuSO4) and sodium hydroxide (NaOH)
adding ammonium hydroxide (NH4OH) 2. Reactions of silver halides
Table 2. Table of reactions of silver halides Page 14
Chemical Engineering Laboratory Reaction Observation Chemical Equation 0.5M potassium chloride (KCl) white precipitate AgNO3 + KCl → AgCl↓ + 0.1M silver nitrate (AgNO3) appeared + KNO3 0.5M potassium chloride (KCl) white precipitate KCl + AgNO3 + NH4OH + 0.1M silver nitrate (AgNO3) appeared → AgCl↓ + KOH +
+ 2M ammonium hydroxide (NH4OH) NH4NO3 0.5M potassium bromide (KBr) white precipitate AgNO3 + KBr → AgBr↓ + 0.1M silver nitrate (AgNO3) appeared + KNO3 0.5M potassium bromide (KBr) white precipitate KBr + AgNO3 + NH4OH + 0.1M silver nitrate (AgNO3) appeared. → AgBr↓ + KOH +
+ 2M ammonium hydroxide (NH4OH) NH4NO3
The experiment matched theoretical data because ion silver (Ag+) could combined with halogen
ions to make precipitations (Figure 5, Figure 7). Different halogen ions made different colors of
precipitation which happened when two liquids were in the reaction of ammonium hydroxide (NH4OH) (Figure 6, Figure 8).
Figure 4. (a) Reaction of potassium chloride
Figure 4. (b) Reaction of the mixture after (KCl) and silver nitrate (AgNO
adding ammonium hydroxide (NH4OH) 3) Page 15
Chemical Engineering Laboratory
Figure 4. (c) Reaction of potassium bromide
Figure 4. (d) Reaction of the mixture after
(KBr) and silver nitrate (AgNO3)
adding ammonium hydroxide (NH4OH)
3. Reactions of hydrogen peroxide (H2O2)
Table 3. Table of reactions of hydrogen peroxide Reaction Observation Chemical Equation
0.1M potassium permanganate (KMnO4) purple solution lost 5 H2O2 + 2 KMnO4 + 3 + 2M sulfuric acid (H2SO4) color, turned gas bubbles H2SO4 → 5O2 + + 3% hydrogen peroxide (H2O2) 2MnSO4 + K2SO4 + 8H2O 0.1M potassium iodide (KI) yellow solution lost H2O2 + H2SO4 + KI → + 2M sulfuric acid (H2SO4) color, purple precipitate H2O + I2 + K2SO4 + 3% hydrogen peroxide (H2O2) appeared 3% hydrogen peroxide (H2O2) black precipitate H2O2 + MnO2 → H2O + manganese dioxide (MnO2) appeared, gas bubbles + O2 + MnO
For tube 1: Sulfuric acid (H2SO4) did not occur when reacting with potassium permanganate
(KMnO4); the solution remains the dark purple color of potassium permanganate (KMnO4).
And after continuing to put hydrogen peroxide (H2O2) into the test tube, potassium Page 16
Chemical Engineering Laboratory
permanganate (KMnO4) solution gradually fades and bubbles due to oxygen (O2) gas (Figure 3. (a)).
For tube 2: Nothing happens when sulfuric acid (H2SO4) reacts with KI. When 3% hydrogen
peroxide (H2O2) was added to the mixture, the solution turned green, then gradually turned
yellow, and a black precipitate appeared (Figure 3. (b)).
For tube 3: there are air bubbles that rise and black precipitate manganese dioxide (MnO2).
Hydrogen peroxide decomposes into water and oxygen upon heating or in the. It combines
with many compounds to form crystalline solids useful as mild oxidizing agents. In most of
its reactions, hydrogen peroxide (H2O2) oxidizes other substances, although it is itself
oxidized by a few compounds, such as potassium permanganate (KMnO4) (Figure 3. (c)).
Figure 5. (a) Reaction of hydrogen peroxide
Figure 5. (b) Reaction of hydrogen peroxide (H (H2O2) in sulfuric acid (H2SO
2O2) in sulfuric acid (H2SO4) with 4) with potassium permanganate (KMnO potassium iodide (KI) 4) Page 17
Chemical Engineering Laboratory
Figure 5. (c) Reaction of hydrogen peroxide (H2O2) with manganese dioxide (MnO2)
4. Reactions of potassium permanganate (KMnO4)
Table 4. Table of reactions of potassium permanganate Reaction Observation Chemical Equation 0.5M sodium sulfite (Na2SO3) purple solution lost 5Na2SO3 + 2KMnO4 + + 2M sulfuric acid (H2SO4) color, turned 3H2SO4 → 5 Na2SO4 +
+ 0.1M potassium permanganate (KMnO4) colorless solution 2MnSO4 + K2SO4 + 3H2O 0.5M sodium sulfite (Na2SO3) purple solution 2KMnO4 + Na2SO3 + + 6M sodium hydroxide (NaOH) turned green 2NaOH → H2O + Na2SO4
+ 0.1M potassium permanganate (KMnO4) solution + K2MnO4 + Na2MnO4 0.5M sodium sulfite (Na2SO3) purple solution lost H2O + 2KMnO4 + + water (H2O) color, black 3Na2SO3 → 2KOH +
+ 0.1M potassium permanganate (KMnO4) precipitate appeared 2MnO2 + 3Na2SO4
The reactions in three tubes did not happen as demonstrable in chemical equations. In tube 1, the
solution changed from purple to colorless due to the formation of manganese sulfate (MnSO4)
(Figure 4. (a)). In tube 2, the solution changed from purple to green due to the formation of Page 18
Chemical Engineering Laboratory manganate ion (MnO 2-
4 ) (Figure 4. (b)). In tube 3, the solution changed from purple to green,
then turned into brown due to the formation of manganese dioxide (MnO2) (Figure 4. (c)). All
three reactions were redox reactions.
Figure 6. (a) Reaction of potassium
Figure 6. (b) Reaction of potassium
permanganate (KMnO4) in sulfuric acid
permanganate (KMnO4) in sodium hydroxide
(H2SO4) with sodium sulfite (Na2SO3)
(NaOH)) with sodium sulfite (Na2SO3)
Figure 6. (c) Reaction of potassium permanganate (KMnO4)
in water with sodium sulfite (Na2SO3)
5. Reactions of ferric ion (Fe3+) and ferrous ion (Fe2+)
Section 1. Reactions of ferric ion (Fe3+)
Table 5. Table of reactions of ferrous ion (3+) Page 19
Chemical Engineering Laboratory Reaction Observation Chemical Equation 0.5M iron trichloride (FeCl3) reddish-brown KOH + FeCl3 → 3KCl + + 2M potassium hydroxide (KOH) precipitate appeared Fe(OH)3↓ 0.5M iron trichloride (FeCl3) reddish-brown 3NH4OH + FeCl3 →
+ 2M ammonium hydroxide (NH4OH) precipitate appeared 3NH4OH + Fe(OH)3↓
Ferric ion (Fe3+) was reacted with residue hydroxide ion (OH-) to form iron (III) hydroxide
Fe(OH)3↓ (Figure 5.A. (a), Figure 5.A. (b)), which was the precipitated product having reddish- brown.
Figure 7.A. (a) Reaction of iron trichloride
Figure 7.A. (b) Reaction of iron trichloride
(FeCl3) and potassium hydroxide (KOH)
(FeCl3) ammonium hydroxide (NH4OH)
Section 2. Reactions of ferrous ion (Fe2+)
Table 6. Table of reactions of ferrous ion (2+) Reaction Observation Chemical Equation 0.5M iron sulphate (FeSO4) green precipitate 2KOH + FeSO4 → K2SO4 + + 2M potassium hydroxide (KOH) appeared Fe(OH)2↓ 0.5M iron sulphate (FeSO4) green precipitate 2NH4OH + FeSO4 →
+ 2M ammonium hydroxide (NH4OH) appeared (NH4)2SO4 + Fe(OH)2↓ Page 20
Chemical Engineering Laboratory
Ferrous ion (Fe2+) was reacted with residue hydroxide ion (OH-) to form iron hydroxide
(Fe(OH)2↓) (Figure 5.B. (a), Figure 5.B. (b)), it was the precipitated product having a dark green
color. Although Iron (II) hydroxide (Fe(OH)2↓) had a white color, having a little bit of oxygen in
the air can make it green (dark or light color depending on concentration).
Figure 7.B. (a) Reaction of iron sulphate
Figure 7.B. (b) Reaction of iron sulphate
(FeSO4) and potassium hydroxide (KOH)
(FeSO4) and ammonium hydroxide (NH4OH)
6. Reactions of aluminum ion (Al3+)
Table 6. Table of reactions of aluminum ion Reaction Observation Chemical Equation
0.5M aluminum sulphate Al2(SO4)3 white precipitate Al2(SO4)3 + 6 NaOH → + 2M sodium hydroxide (NaOH) appeared 2Al(OH)3↓ + 3Na2SO4
0.5M aluminum sulphate Al2(SO4)3 white precipitate Al2(SO4)3 + 6NaOH + + 2M sodium hydroxide (NaOH) appeared, then a part of 6HCl → 2AlCl3 + + 2M hydrochloric acid (HCl) precipitate dissolved to 3Na2SO4 + 6H2O form a colorless solution
0.5M aluminum sulphate Al2(SO4)3 white precipitate Al2(SO4)3 + 8NaOH → + 2M sodium hydroxide (NaOH) appeared, then precipitate 2NaAlO2 + 3Na2SO4 + + 2M sodium hydroxide (NaOH) dissolved to colorless 4H2O Page 21
Chemical Engineering Laboratory
When 0.5M aluminum sulfate (Al2(SO4)3) with 2M sodium hydroxide (NaOH) (Figure 6. (a))
were added to test tubes 1 and 2, after the reaction, observing the appearance of a white colloidal
precipitate of aluminum hydroxide (Al(OH)3) red in the solution.
Then, giving the results of test tube 1 reacting with 2M hydrochloric acid (HCl), the results were
observed that white solid of aluminum hydroxide (Al(OH)3) gradually dissolved in solution (Figure 6. (b)).
For test tube 2, which continued to react with 2M sodium hydroxide (NaOH), the phenomenon
of this test tube was a precipitate that gradually dissolved to form a transparent solution (Figure 6. (c)).
Figure 8. (a) Reaction of aluminum sulphate
Figure 8. (b) Reaction of mixture aluminum
Al2(SO4)3 and sodium hydroxide (NaOH)
sulphate Al2(SO4)3 and sodium hydroxide
(NaOH) after added hydrochloric acid (HCl) Page 22
Chemical Engineering Laboratory
Figure 8. (c) Reaction of mixture aluminum sulphate Al2(SO4)3 and sodium hydroxide (NaOH)
after added more sodium hydroxide (NaOH) 7. Flame test
Table 7. Table of flame testing Reaction Observation Chemical Equation Lithium chloride (LiCl) red flame appeared Sodium chloride (NaCl) yellow flame appeared Potassium chloride (KCl) purple flame appeared Calcium dichloride (CaCl2) red orange flame appeared Barium chloride (BaCl2) orange flame appeared
The experiment matched theoretical data because the flame test for each element was different as
ions of each element had a specific feature based on their emission spectrum and cause the fire to
emit light at a characteristic color. Page 23
Chemical Engineering Laboratory
Figure 9. (a) Red flame happened when
Figure 9. (b) Yellow flame happened when
burning lithium chloride (LiCl) burning sodium chloride (NaCl)
Figure 9. (d) Red orange flame happened
Figure 9. (c) Purple flame happened when
when burning calcium dichloride (CaCl2)
burning potassium chloride (KCl)
Figure 9. (e) Orange flame happened when burning barium chloride (BaCl2) Conclusion
This experiment was conducted and achieved the objectives, which were chemical reaction
performances such as synthesis, decomposition, single displacement, double displacement, and Page 24
Chemical Engineering Laboratory
combustion. Results from experiments were consistent with the theory. The findings
demonstrated that the precipitates which formed from displacement reactions in reactions of
copper ion (Cu2+), silver halides, ferric ion (Fe3+) and ferrous ion (Fe2+) had many colors and can
probably be dissolved by the concentration of reactants. The consequences of reactions of
hydrogen peroxide (H2O2 ) or potassium permanganate (KMnO4) were from oxidation-reduction
reactions. The test on acid and base in reactions of ions Fe2+, Fe3+ and Al3+ indicated clearly the
relationship between precipitates and acid or base. Basic types of reactions were illustrated in
this experiment that can be found in procedures. The flame test brought the range of colors that
were created when various salts come into contact with a flame. Besides, in this experiment, the
executor had the task of distinguishing the different reactions and precipitates, which were based
on forms, colors, and other characteristics. In the future, identifying the product of a reaction can
be easier as well as determining the kind of reaction. Some chemical professions can depend on
the technique of flame test to recognize the salt. Page 25
Chemical Engineering Laboratory Reference
[1] Anne Marie Helmenstine, P. D. (n.d.). A description of synthesis reactions and some examples. ThoughtCo.
[2] Introductory,conceptual,andGobChemistry. Chemistry LibreTexts. (2022, June 11).
[3] Takeonlinecourses.earn collegecredit.ResearchSchools,Degrees &Careers. Study.com |
Take Online Courses. Earn College Credit. Research Schools, Degrees & Careers. (n.d.).
[4] Khan Academy. (n.d.). Doublereplacementreactions(doubledisplacement)(article). Khan Academy. Retrieved from
[5] Libretexts. (2022, August 8). 11.6: Combustionreactions. Chemistry LibreTexts.
[6] The flame test - soinc.org. The Flame Test. Lange's Handbook of Chemistry, 8th Edition,
Handbook Publishers Inc., 1952.




