








Preview text:
lOMoARcPSD|364 906 32
VIETNAM NATIONAL UNIVERSITY – HO CHI MINH
CITY INTERNATIONAL UNIVERSITY CHEMISTRY LAB REPORT
EXPERIMENT 1: CHEMICAL REACTIONS
Instructor: Lê Trần Hồng Ngọc Name: Võ Thị Thảo Vy ID: BTBTIU22187 Group: 16
Academic year: 2023-2024 I/ Introduction
Chemical reactions play an essential role in human activities, particularly in creating a
new substance to produce many types of materials that serve human need. In one
chemical reaction is the participation of substances called reactants which are 1 lOMoARcPSD|364 906 32
transformed into different substances which are considered as products and they appear
with various characteristics. In experiments 1, we practiced and observed some types of
chemical reactions. Chemical changes in these reactions can be the the transformation of
color, the formation of a solid, the release of gas, and the production of heat and light.
Generally, chemical reactions are represented in five types of reactions: synthesis,
decomposition, single displacement, double displacement, and combustion. II/ Objectives
• To perform different types of chemical reactions (acid-base, precipitate, gas
forming, complex compound forming, and oxidation-reduction reactions).
• To identify some of the products in these reactions and describe the chemical changes.
• To write and balance the chemical equations for the reactions observed. III/ Materials and equipment 1/. Materials 0.5M CuSO4 0.1M KMnO4 2M NaOH 2M H2SO4 2M NH4OH 3% H2O2 0.5M KCl 0.1M KI 0.1M AgNO3 MnO2 0.5M KBr 0.5M Na2SO3 6M NaOH CaCl2 2M HCl 2M KOH Distilled water BaCl2 LiCl 0.5M FeSO4 0.5M FeCl3 0.5M Al2(SO4)3 2/. Equipment Test tubes Alcohol lamp Test tube rack Looped wire Test tube holders Distilled water bottle 2 lOMoARcPSD|364 906 32 Beakers
IV/ Procedure, result, and discussion 1/ Reaction of Cu 2+ Procedure:
Step 1: Put 10 drops 0.5M CuSO4 into each two test tube.
Step 2: Put 10 drops 2M NaOH and 10 drops 2M NH4OH into the first test tube and
the second test tube, respectively. Then observe the phenomenon.
Step 3: After a few seconds, continue to put 10 drops 2M NaOH and 10 drops 2M
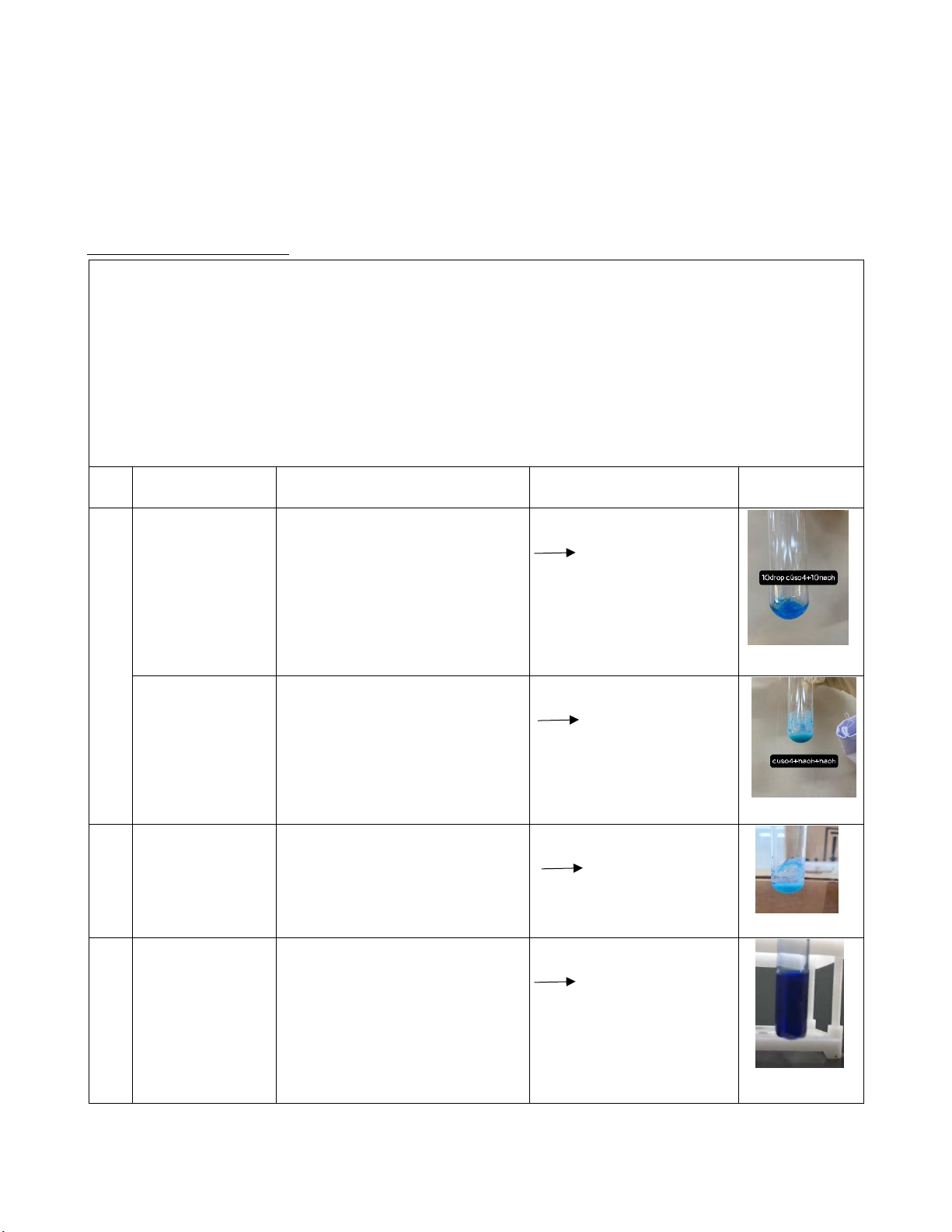
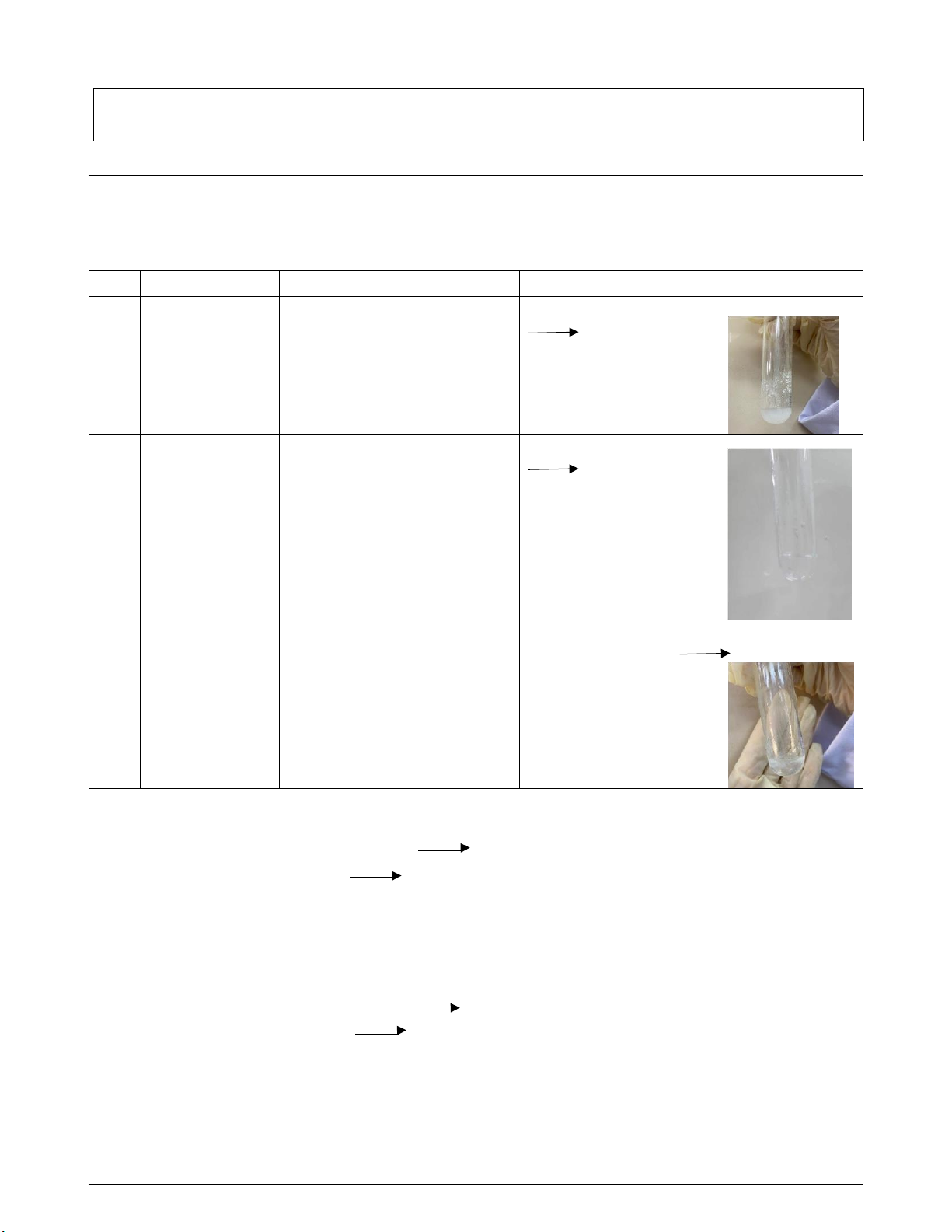
NH4OH into the first test tube and the second test tube, respectively. Then observe the phenomenon. Reaction Observation Chemical equation Image #1 0.5M CuSO4 There appears blue CuSO4 + 2NaOH + 2M NaOH precipitation and colloidal Na2SO4 + phenomena were formed Cu(OH)2 0.5M CuSO4
The color of the solution is CuSO4 + 2NaOH + 2M NaOH lighter than the previous Na2SO4 + + 2M NaOH reaction. However, the Cu(OH)2 precipitation is not disappeared.
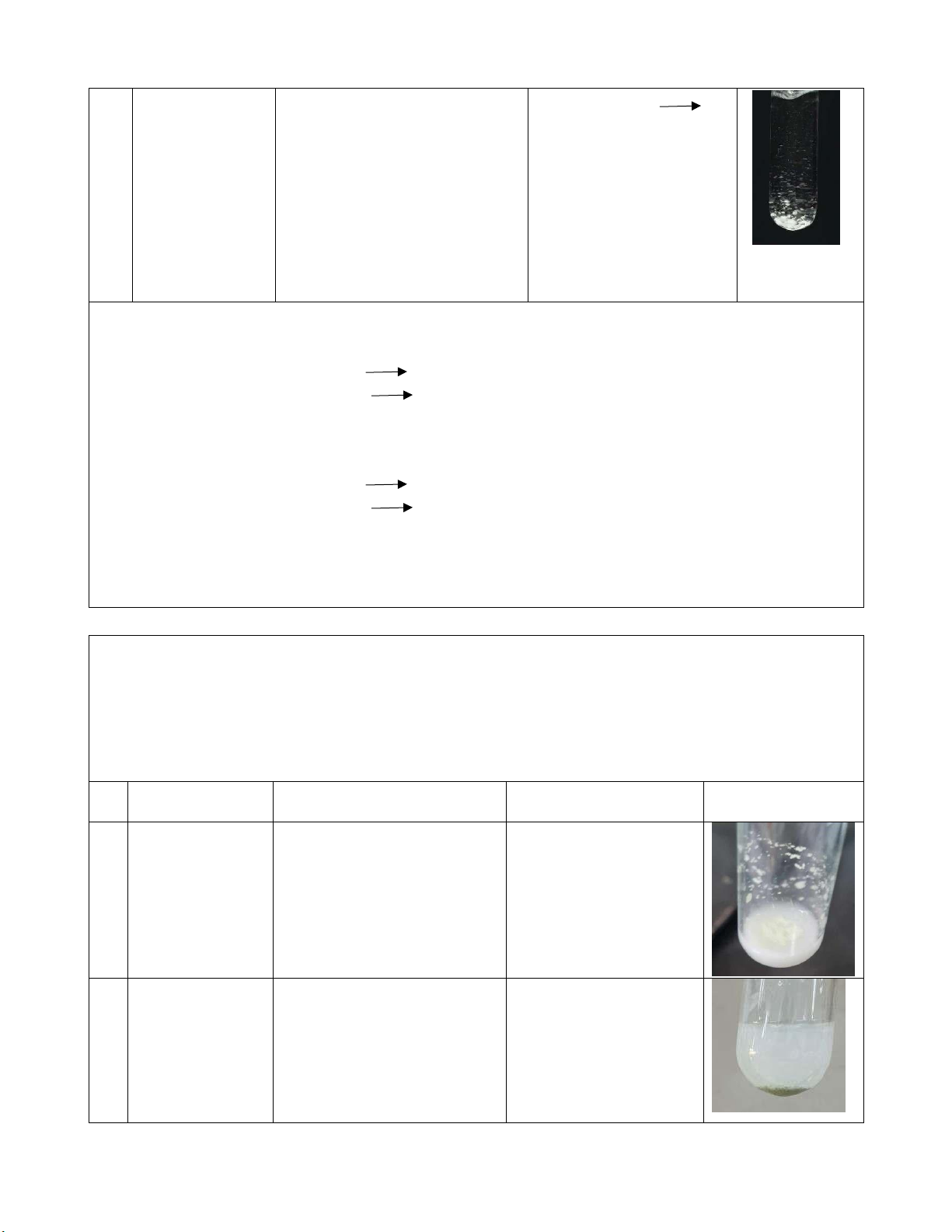
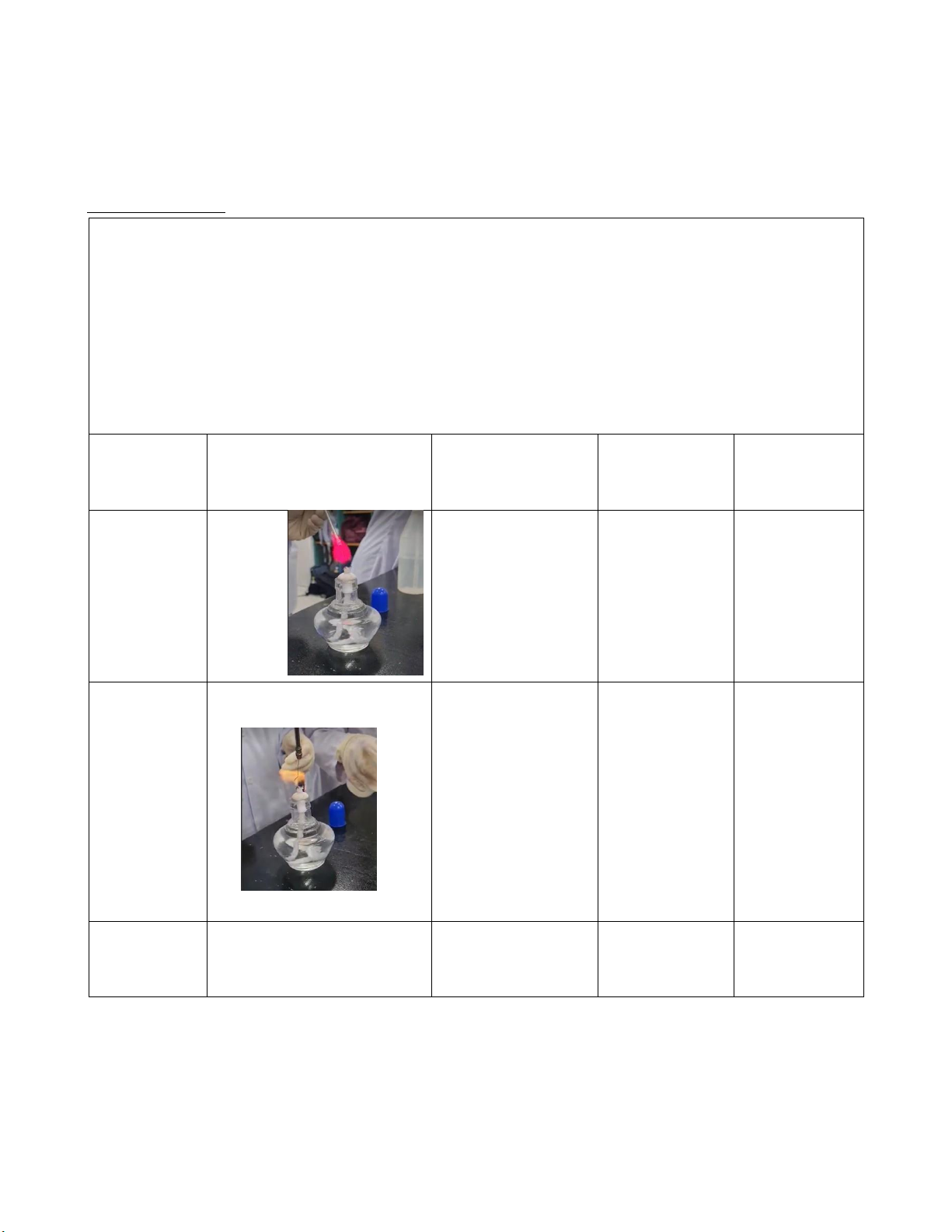
#2 0.5M CuSO4 + There appears blue CuSO4 + 2NH4OH 2M NH4OH precipitation. (NH4)2SO4 + Cu(OH)2 0.5M CuSO4 The color of the solution Cu(OH)2 + 4NH4OH
+ 2M NH4OH becomes darker than the 4H2O +
+ 2M NH4OH previous reaction. The [Cu(NH3)4](OH)2 precipitation is dissolved. 3 lOMoARcPSD|364 906 32 Comments: #1: CuSO4 is dissociated: CuSO4 Cu2+ + SO 2 4 NaOH is dissociated: NaOH Na+ + OH-
Cation Cu2+ and Anion OH- combine to each other then form the blue precipitation Cu(OH)2.
When adding more 2M NaOH into the first test tube, the phenomenon proves that the
excess of NaOH does not influence the reaction. #2: CuSO 2 4 is dissociated: CuSO4 Cu2+ + SO4 NH4OH is dissociated: NH + 4OH NH4 + OH-
Cation Cu2+ and Anion OH- combine to each other then form the blue precipitation Cu(OH)2.
When adding more 2M NH4OH into the second test tube, the phenomenon proves that
there is another reaction happens. More specifically, after the precipitation Cu(OH)2 is
formed, it continue to reacts with excess NH4OH and form the complex [Cu(NH3)4] (OH)2.
2/ Reactions of Silver halides
Section 1: Reactions of Potassium Chloride (KCl) Procedure:
Step 1: Put 10 drops 0.5M KCl into each two test tube.
Step 2: Continue to put 10 drops 0.1M AgNO3 into each two test tube.
Step 3: Put 10 drops 2M NH4OH into one of two test tubes. Labeling this test tube is
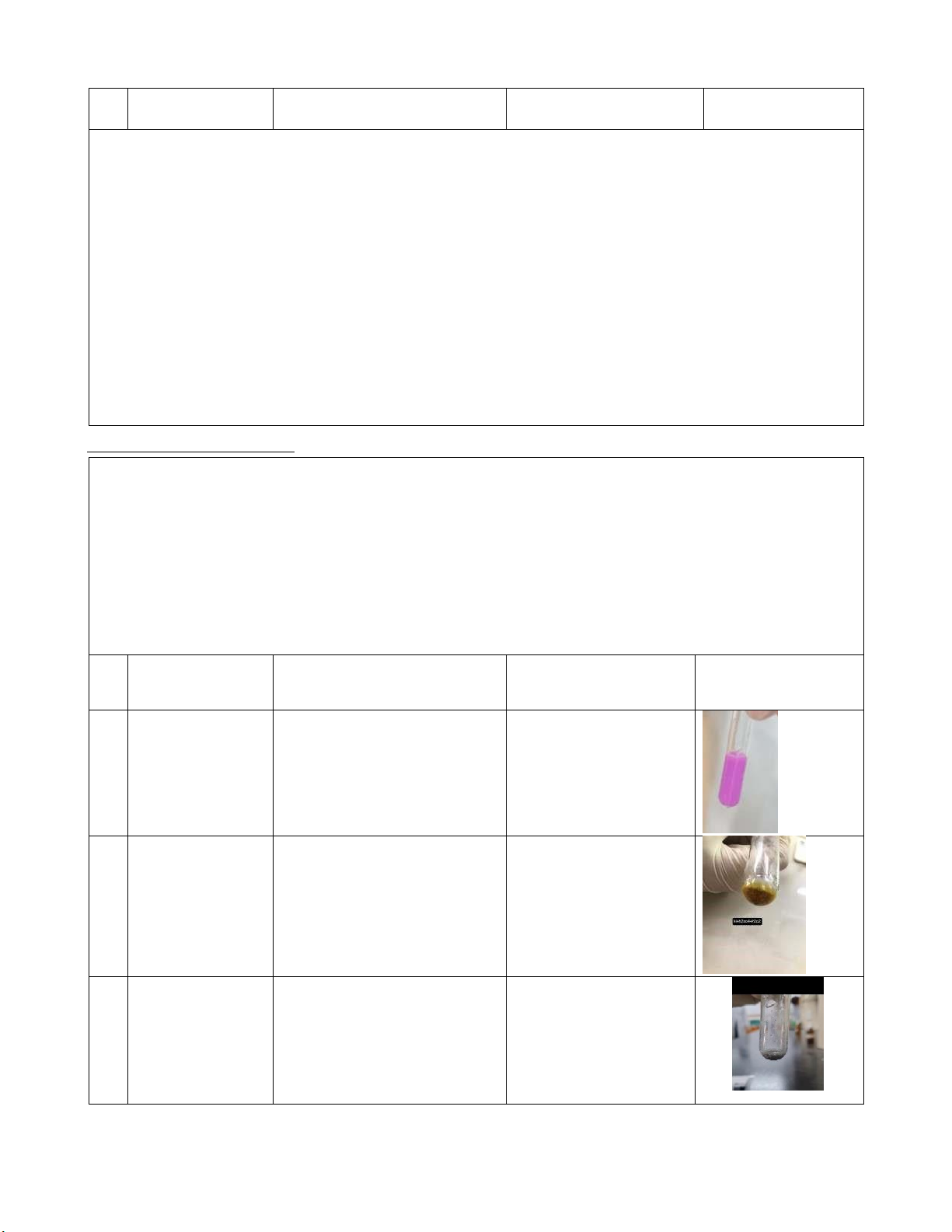
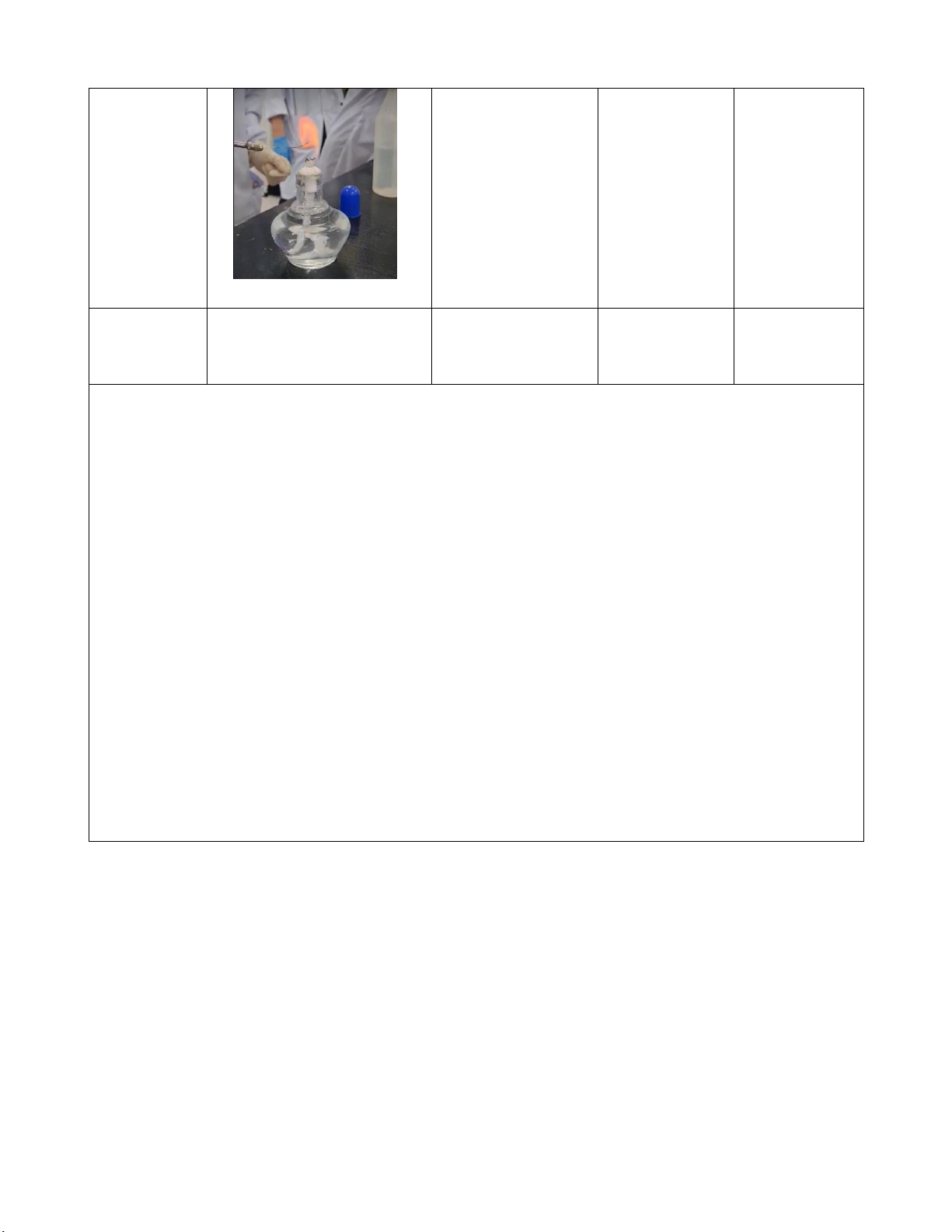
#2, the other is #1. Observe the phenomenon. Reaction Observation Chemical equation Image #1 0.5M KCl + There appears white KCl + AgNO3 0.1M AgNO3 precipitation. KNO3 + AgCl 4 lOMoARcPSD|364 906 32 #2 0.5M KCl + There appears white KCl + AgNO3 0.1M AgNO precipitation after the 3 KNO3 + AgCl AgCl + 2M NH
reaction between KCl and + 2NH OH → 4OH 4 AgNO3. [Ag(NH3)2]Cl + H2O When put 2M NH4OH solution into above solution, the white precipitation disappeared. Comments: #1: KCl is dissociated: KCl K+ + Cl- AgNO - 3 is dissociated: AgNO3 Ag+ + NO3
Cation Ag+ and Anion Cl- combine to each other then form the blue precipitation AgCl. #2: KCl is dissociated: KCl K+ + Cl- AgNO - 3 is dissociated: AgNO3 Ag+ + NO3
Cation Ag+ and Anion Cl- combine to each other then form the blue precipitation AgCl.
However, when adding more NH4OH, it continues to react with AgCl to form another
substances which are [Ag(NH3)2]Cl and H2O, the white precipitation disappeared.
Section 2: Reactions of Potassium Bromide (KBr) Procedure:
Step 1: Add 10 drops of 0.5M KBr into two test tubes ( test tube #1 and test tube #2).
Step 2: Add 10 drops of 0.1M AgNO3 into both test tubes.
Step 3: Add 10 drops of 2M NH4OH into test tube #2
Step 4: Mix tubes gently and wait at least 2 minutes, then observe the phenomenon. Reaction Observation
Chemical equation Image #1 The light-yellow precipitate was formed. 0.5M KBr + AgNO3 + KBr → 0.1M AgNO3 AgBr + KNO3 #2 0.5M KCl + The light yellow AgNO +KBr → 3
0.1M AgNO3 precipitate was formed. AgBr + KNO3 + 2M NH4OH Then after adding NH4OH, the light AgBr + 2NH OH → 4 yellow precipitate 4[Ag(NH3)2]Br + 5 lOMoARcPSD|364 906 32 started to dissolve H2O Comments: #1:
KBr dissociates to form ion K+ and Br-
In the solution, ion Br- combines with ion Ag+ to form AgBr precipitate which is yellow
because it has low solubility. #2:
KBr dissociates to form ion K+ and Br-.
In the solution, ion Br- combines with ion Ag+ to form AgBr precipitate which is yellow.
After adding NH4OH, The precipitate AgBr reacts with NH4OH to create the
[Ag(NH3)2]Br, which dissolves completely in the solution. 3/ Reactions of H2O2 Procedure:
Step 1: Add 1 drop of 0.1M KMnO4; 5 drops of 0.1M KI; 5 drops of 3% H2O2 into test
tube #1, #2, and #3, respectively.
Step 2: Add 5 drops of 2M H2SO4 into both test tube #1 and #2. Then add a pinch of MnO2 into test tube #3.
Step 3: Add 5 drops of 3% of H2O2 into both test tube #1 and #2.
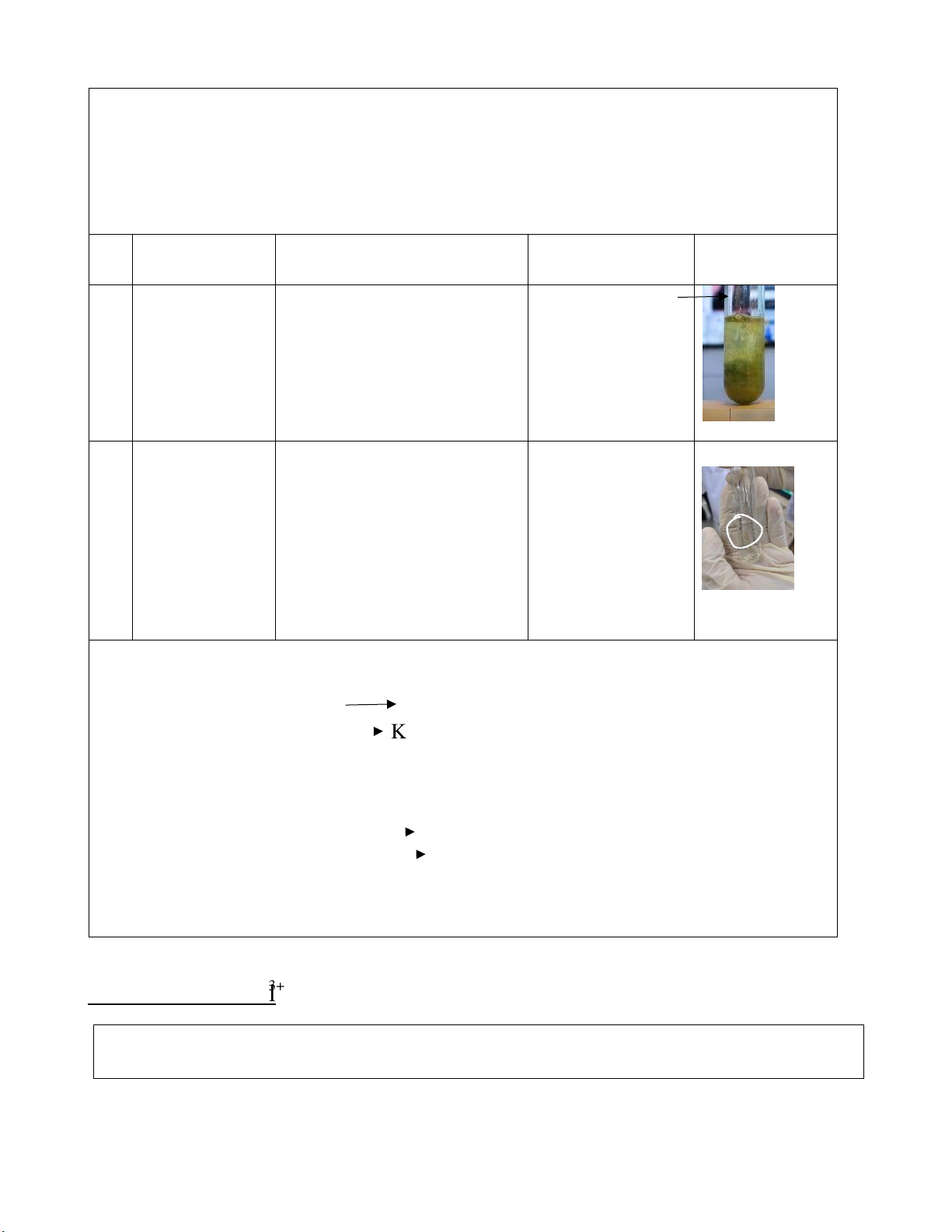
Step 4: Mix tubes gently and wait at least 2 minutes, then observe the phenomenon. Reaction Observation Chemical Image equation #1 The solution transforms 2KMnO4 + 3H2SO4
0.1M KMnO4 + lightly purple then the + 5H2O2 → 8H2O + 2M H2SO4 + air bubbles appear. 2MnSO 3% H 4 + 5O2 + 2O2 K2SO4 #2 After adding H2O2 the
0.1M KI + 2M color changed into 2KI + H2SO4 + H → 2SO4 + 3% lightorange color and H2O2 K2SO4 + H2O2 purple precipitate 2H2O + I2 appeared. #3 3% H2O2 + A After adding MnO2, the H2O2 → O2 + H2O pinch of release of gas and heat MnO2 appeared and the 6 lOMoARcPSD|364 906 32 blackgray solid didn’t dissolve. Comments: #1:
In this reaction, H2O2 reacts with H2SO4 to form O2:
2H2O2 + 2H2SO4 → 2H2O +2SO2 +O2
Oxygen on the previous reaction continues to react with KMnO4. This chemical is
dissociated to form Mn2+ and Mn3+:
2H2O2 + 6H+ + 2MnO4- → 5H2O + 2Mn2+ + 8H2O + 5O2 #2:
In this reaction, firstly is the reaction between KI and H2SO4: 2KI + H2SO4 → K2SO4 + 2HI
Next, HI reacts with H2O2 to form I2 gas and H2O: 2HI + H2O2 → I2 + 2H2O
So that the precipitate in this reaction is I2. #3:
H2O2 in this reaction is decomposed and MnO2 plays a role as catalyst. So that MnO2
promote the speed of the reaction to form O2 (gas) so we can observe many bubbles on the test tube. 4/ Reactions of KMnO4 Procedure:
Step 1: Add 10 drops of 0.5M Na2SO3 into each three test tubes #1, #2 and #3
Step 2: Add 5 drops of 2M H2SO4 into test tubes #1; 5 drops of 6M NaOH into test
tube #2; 5 drops of distilled water into test tubes #3
Step 3: Add 5 drops of 0.1M KMnO4 into test tube #1, #2, and #3.
Step 4: Mix tubes gently and observe the phenomenon. Reaction Observation Chemical equation Image
#1 0.5M Na2SO3 The solution changed to 2KMnO4 + 3H2SO4 + + 2M H2SO4 light purple color and no 5Na2SO3 → 2MnSO4 + 0.1M participate formed. + K2SO4 + 3H2O + KMnO4 5Na2SO4 7 lOMoARcPSD|364 906 32
#2 0.5M Na2SO3 After putting the three 2KMnO4 + 2NaOH + + 6M NaOH chemicals into the test Na2SO3 → KOH + + 0.1M tube, the solution turned Na2MnO4 + Na2SO4 KMnO4 dark green. After a few + H2O + MnO2 seconds, it turned to brown, then the brown precipitate appeared.
#3 0.5M Na2SO3 The solution changed to 2KMnO4 + 3Na2SO3 + distilled dark-brown color. The +H2O → 2MnO2 +
water + 0.1M brown precipitate 3Na2SO3 + 2KOH KMnO4 appeared. Comments: #1:
KMnO4 is a chemical that can oxidize many substances in the acid environment. When
adding KMnO4 into the solution includes Na2SO3 and H2SO4, KMnO4 reacts with
H2SO4: 4KMnO4 + 6H2SO4 → 2K2SO4 + 4MnSO4 + 6H2O + 5O2 Na2SO3 is oxidized to form Na2SO4:
Na2SO3 + H2SO4 → Na2SO4 + SO2 + H2O
H2SO4 in this reaction plays a role as a oxidized substance that promotes the oxidized process. #2:
In this reaction, there is a chain of reactions below: KMnO 2+
4 reacts with NaOH to form K2MnO4 and H2O, ion Mn7+ is reduced
to Mn : 3KMnO4 + 2NaOH → K2MnO4 + MnO2 + 2H2O
KMnO4 continues to react with Na2SO3 to form MnO2, Na2SO4, and Na2MnO4: 3KMnO O → 3MnO 4 + 5Na2SO3 + 2H2 2 + 5Na2SO4 + 2Na2MnO4 + 2KOH The precipitate here is MnO2. #3:
In H2O environment, Na2SO3 is oxidized to form Na2SO4, KMnO4 is reduced to form MnO2 (the brown precipitate).
5/ Reactions of Fe 2+ and Fe 3
Section 1: Ferric ion (Fe3+) 8 lOMoARcPSD|364 906 32 Procedure:
Step 1: Put 10 drops 0.5M FeCl3 into each two test tubes
Step 2: Put 5 drops 2M KOH and 5 drops 2M NH4OH into the first and the second test tubes, respectively.
Step 3: Observe the phenomenon. Reaction Observation Chemical equation Image
#1 0.5M FeCl3 + The solution becomes 2M KOH red-brown and the FeCl3 + 3KOH precipitation also has Fe(OH)3 + 3KCl red-brown color.
#2 0.5M FeCl3 + There appears red-brown FeCl3 + 3NH4OH 2M NH4OH precipitation on the 3NH4Cl + reaction. Fe(OH)3 Comments: #1:
FeCl3 is dissociated: FeCl3 → Fe3+ + Cl-
KOH is dissociated: KOH → K+ + OH-
The cation Fe3+ and the anion OH- combine together to form the precipitation Fe(OH)3. #2:
FeCl3 is dissociated: FeCl3 → Fe3+ + Cl- NH OH → NH4+ 4OH is dissociated: NH4 + OH-
The cation Fe3+ and the anion OH- combine together to form the precipitation Fe(OH)3.
Section 2: Ferrous ion (Fe2+) 9 lOMoARcPSD|364 906 32 Procedure:
Step 1: Put 10 drops 0.5M FeSO 4 into each two test tubes
Step 2: Put 5 drops 2M KOH and 5 drops 2M NH 4 OH into the first and the second test tubes, respectively.
Step 3: Observe the phenomenon. Reaction Observation Chemical Image equation
#1 0.5 M FeSO 4 + The dark green precipitate 2 M KOH is formed after keeping it FeSO 4 + 2KOH on sphere for some K 2 SO 4 + minutes. Fe(OH) 2 #2 0.5M
FeCl 3 + There appears red-brown 2 M NH FeSO 4 +2 NH 4 OH 4 OH precipitation on the reaction. -> (NH 4 ) 2 SO 4 + Fe(OH) 2 Comments: #1: FeSO 4 is dissociated: FeSO 4 Fe 2+ + SO 4 2-
KOH is dissociated: KOH K + + OH -
The cation Fe 2+ and the anion OH - combine together to form the precipitation Fe(OH) 2 . #2:
FeSO 4 is dissociated: FeSO 4 Fe 2+ + SO 4 2-
NH 4 OH is dissociated: NH 4 OH NH 4+ + OH -
The cation Fe 2+ and the anion OH - combine together to form the precipitation Fe(OH) 2 . 6/ Reactions of Al 3+ Procedure:
Step 1: Put 10 drops Al2(SO4)3 into each two test tubes. 10 lOMoARcPSD|364 906 32
Step 2: Continue to put 5 drops 2M NaOH into each two test tubes. Observe the phenomenon.
Step 3: Put 20 drops 2M HCl and 20 drops 2M NaOH into the first and the second test tube, respectively.
Step 4: Observe the phenomenon. Reaction Observation Chemical equation Image #1 0.5M
The white precipitation is Al2(SO4)3 + NaOH and Al2(SO4)3 + formed. Na2SO4 + #2 2M NaOH Al(OH)3 #1 0.5M After the white Al(OH)3 + 3HCl
Al2(SO4)3 2M precipitation is formed AlCl3 + 3H2O
NaOH + 2M by the reaction between HCl Al2(SO4)3 + NaOH, when adding HCl solution, the white precipitation disappears, a no-color solution is form. #2 0.5M
The white precipitation Al(OH)3 + NaOH Al2(SO4)3 + disappears. NaAlO2 + 2H2O 2M NaOH + 2M NaOH Comments: #1 Al 2-
2(SO4)3 is dissociated: Al2(SO4)3 Al3+ + SO4
NaOH is dissociated: NaOH Na+ + OH-
The cation Al3+ and the anion OH- combine together to form the precipitation Al(OH)3.
When adding more 2M HCl solution, it continues to react with Al(OH)3 to form new
substances which are AlCl3 (due to ion Al3+ combined to Cl-) and H2O. The white
precipitation which is Al(OH)3 is disappeared. #2: Al 2-
2(SO4)3 is dissociated: Al2(SO4)3 Al3+ + SO4
NaOH is dissociated: NaOH Na+ + OH-
The cation Al3+ and the anion OH- combine together to form the precipitation Al(OH)3.
When adding more 2M NaOH solution, it continues to react with Al(OH)3 to form new 11 lOMoARcPSD|364 906 32 substances which are NaAlO -
2 (due to ion Na+ combined AlO2 ) and H2O. The white
precipitation which is Al(OH)3 is disappeared. 7/ Flame tests Procedure:
Step 1: Prepare 5 test tubes containing LiCl, NaCl, KCl, CaCl2, and BaCl2 solutions.
Step 2: Light the burner
Step 3: We clean the loop with distilled water.
Step 4: Dip a looped into one of the solutions supplied.
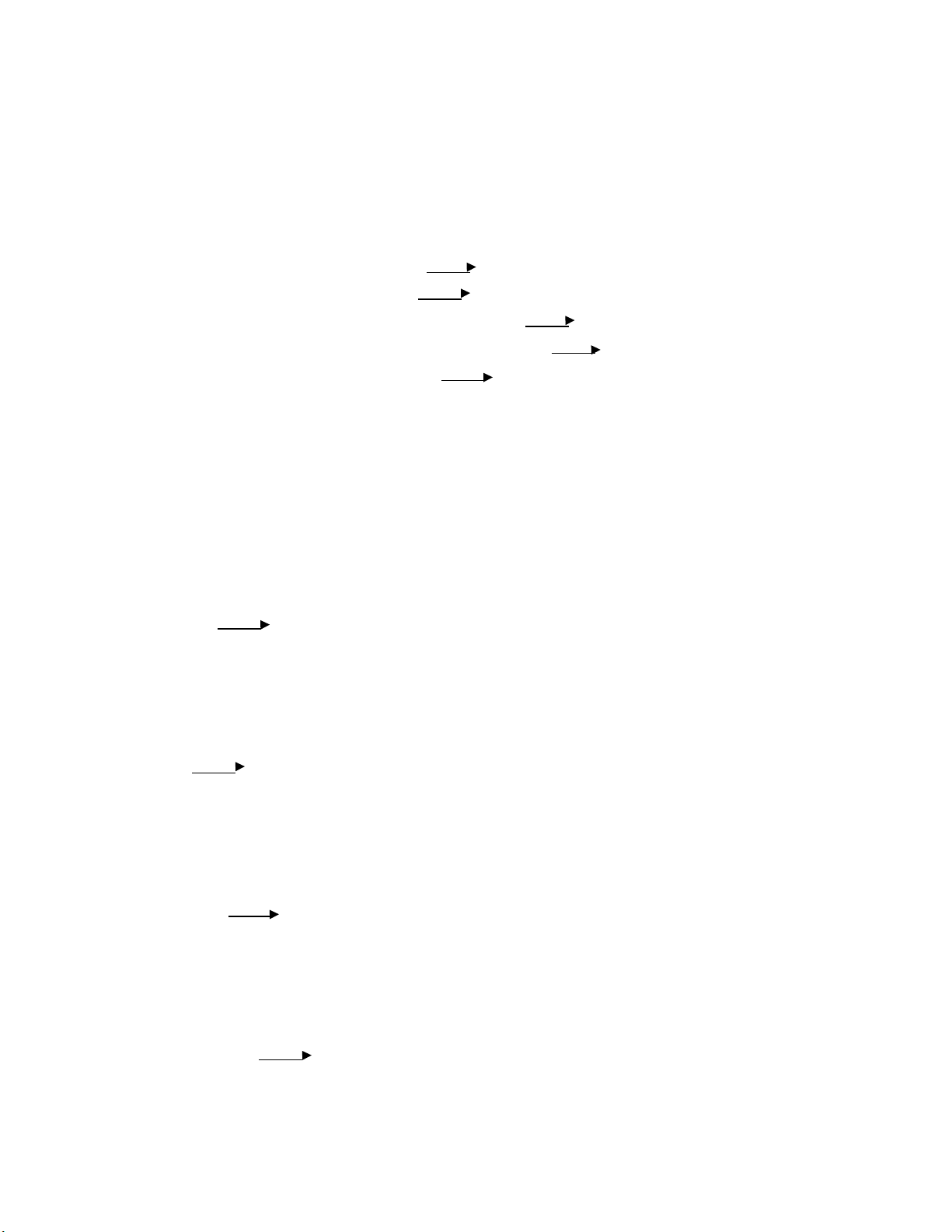
Step 5: Hold it in the flame, after saw the change in the dominant flame color, record it. Wavelength Frequency Photon Solution Dominant flame color (nm) (s−1) energy (J) LiCl 701 4.28 x 1014 2.83 x 10-19 Red NaCl 587 5.11 x 1014 3.39 x 10-19 Yellow KCl Blue Violet 455 6.6 x 1014 4.37 x 10-19 12 lOMoARcPSD|364 906 32 CaCl2 609 4.93 x 1014 3.27 x 10-19 Orange BaCl2 Light Green 577 5.2 x 10-9 3.45 x 10-19 Comments:
We observe the color of the fire when heating LiCl, NaCl, KCl, CaCl2 and BaCl2. As
can be seen, the color when heating them vary mostly due to the different rates of
electrons in different substances.
These are the two equations to calculate the table above. 1. C = λ x v
C: the speed of light (3 x 108 m/s) λ: the wavelength (nm) v: frequency (s-1) 2. Ephoton = h x v
Ephoton: the energy per photon (J) h:
(Planck’s constant) 6.626 x 10-34 (J.s) V/ Suggested questions
1/. What are the purposes of today’s lab report?
The target of Experiment 1 is:
• Doing all the chemical reactions consists of acid-base, precipitation, gas forming,
complex compound forming and oxidation-reducing reactions.
• Analyzing the results of these reactions (the products...) and discussing the chemical changes.
• Complete the chemical equation and balance them for the reactions observed. 13 lOMoARcPSD|364 906 32
2/. What is a chemical reaction?
Chemical reaction is the process of converting a set of substances (the reactants) into
another set of substances (the products). This reaction is related to the changes of the
position of the electrons in forming and breaking the chemical bonds between atoms.
3/. Please give examples of different types of chemical reactions.
• Synthesis reaction: CaO + H2O Ca(OH)2
• Decomposition reaction: 2H2O 2H2 + O2
• Single displacement reaction: 2K + MgCl2 2KCl + Mg
• Double displacement reaction: KNO3 + AlCl3 Al(NO3)3 + KCl
• Combustion reaction: CH4 + 2O2 CO2 + 2H2O
4/. What are observable signs when chemical reaction occur?
The five conditions of chemical changes: color change, formation of a precipitate,
formation of a gas, odor change temperature change.
5/. What is a synthesis reaction? Give an example.
A synthesis reaction is the process in which two or more reactants combine to form a single product. Ex: 2H2 + O2 2H2O
6/. What is a decomposition reaction? Give an example.
In a decomposition reaction, molecules or compounds break down into two or more than
two simpler chemically new substances. Ex: CaCO3 CaO + CO2
7/. What is a single displacement reaction? Give an example.
Single displacement reaction is a reaction in which one element is substituted for
another element in a compound. Ex: Zn + 2HCl ZnCl2 + H2
8/. What is a double displacement reaction? Give an example.
Double displacement reaction is two aqueous ionic compounds exchange their ions
(mostly cations) and produce two new compounds. Ex: KBr + AgNO3 KNO3 + AgBr
9/. What is a combustion reaction? Give an example. 14 lOMoARcPSD|364 906 32
A combustion reaction is a reaction in which a substance reacts with oxygen gas,
releasing energy in the form of light and heat. Ex: C + O2 CO2
10/. Please name all of the experiments that you will do in today’s lab work. 1. Reactions of Cu2+
2. Reactions of silver halides 3. Reactions of H2O2 4. Reactions of KMnO4 5. A. Reactions of Fe3+ B. Reactions of Fe2+ 6. Reactions of Al3+ 7. Flame test
11/. What are molarity and normality?
Molarity is defined as the number of moles of solute per liter of solution.
Molarity = Moles of solute/Volume of solution in liter
Normality is defined as the number of equivalents per liter of solution.
Normality = Number of gram equivalents of solute/Volume of solvent in liter
12/. What is the equation that shows the relationship between wavelength, frequency,
and speed of an electromagnetic wave?
The relationship between wavelength, frequency, and speed of an electromagnetic wave
is represented by the equation: Where: 15 lOMoARcPSD|364 906 32
C is the speed of light (3.108 m/s)
λ is the wavelength (nm) v is frequency IV/ Conclusion
The chemical reaction contributes an important role in all activities of all organisms. In
experiment 1, we are learn and observe many types of chemical reaction and identify
their products by the changes of physical properties. We can observe the chemical
changes by some changes such as colors, states of matter, bubbles…The five general
types of reactions are synthesis, decomposition, single displacement, double displacement, and combustion. 16 lOMoARcPSD|364 906 32 -The End- 17
Document Outline
- I/ Introduction
- II/ Objectives
- IV/ Procedure, result, and discussion
- V/ Suggested questions
- IV/ Conclusion




