











Preview text:
International University, Vietnam National University - HCMC 1 CHEMISTRY LABORATORY REPORT
EXPERIMENT 1: CHEMICAL REACTIONS
Group: 3 Section: 1 Date: 14/07/2022 Group members: Seq. Full name Student ID % Signatur Score contribu e tion (total = 100%) 1 Phạm Vũ Thắng BTCEIU21009 20 2 Nguyễn Thị Anh Thư BTBTIU21156 20 3 Hoàng Nguyễn Quỳnh BTBTIU21137 20 Khanh 4 Nguyễn Ngọc Bảo Hân BTBTIU21164 20 5 Đinh Ngọc Vân Châu BTBTIU21042 20 Total score: _______/100
International University, Vietnam National University - HCMC 2 CHEMISTRY LABORATORY I. Introduction
Since the beginning of time, chemical reactions have been the origin of practically all the matter.
Chemical reactions are now a vital feature of technology, culture, and life itself. In a chemical reaction,
the atoms that make up the reactants are rearranged to produce products, which are substances with
different properties. In this experiment, we will carry out and observe a variety of events to see if
chemical reactions have occurred. A change in properties that can be seen in eyes, such as a change in
color, condition, physicality, temperature differential, and others (for example: the formation of a solid,
the release of gas and the production of heat and light…), can be counted among the observable
indications of a chemical reaction. Sort these reactions into one of the five categories of typical
reactions: synthesis, breakdown, single displacement, double displacement, and combustion.
In this report, we will present and discuss seven main experiments that we did by
- Performing different types of chemical reactions
- Identifying some of the products in these reactions by identifying the chemical changes of them.
- Writing and balancing the chemical equations for the reactions observed.
II. Materials and Methods * Chemicals and Equipments Equipments Three 250ml Beaker One Crucible Tongs Three Test Tubes One Bunsen Burner One Disposable Pipet One Flame Test Loop Chemicals 0.5M CuSO4 0.1M KMnO4 2M NaOH 6M NaOH 2M NH4OH Distilled Water 0.5M KCl 0.5M FeCl3 0.1M AgNO3 2M KOH 0.5M KBr 0.5M Al₂(SO₄ )₃ 2M H2SO4 BaCl₂ 3% H2O2 LiCl 0.1M KI NaCl
International University, Vietnam National University - HCMC 3 CHEMISTRY LABORATORY MnO2 CaCl₂ 0.5M Na2SO3 * Procedure: 1. Reactions of Cu2+
Step 1: Prepare 2 clean test tubes
Step 2: Drip 10 drops of 0.5M CuSO to each tubes 4
Step 3: Add 10 drops of 10M Naoh to the first tube and 10 drops of 2M NH OH to the second one. 4
Step 4: Mix tubes gently and observe the current status of two tubes
Step 5: Then, add 10 drops of each chemical to each tube sequentially.
Step 6: Mix tubes gently and observe the result.
2. Reactions of Silver halides
Section 1: Reactions of Potassium Chloride (KCl)
Step 1: Prepare two clean test tubes
Step 2: Add 10 drops of 10 drops of 0.5M KCL to each one.
Step 3: Then, drip 10 drops of 0.5M AgNO to each tube. 3
Step 4: Add 10 drops 2M NH OH to the second tube only 4
Step 5: Mix tubes gently and observe the current status of two tubes
Section 2: Reactions of Potassium Bromide (KBr)
Step 1: Prepare 2 clean test tubes
Step 2: Then, drip 10 drops of 0.5M AgNO to each tube. 3
Step 3: Add 10 drops 2M NH OH to the second tube only 4
Step 4: Mix tubes gently and observe the result. 3. Reactions of H2O2
Step 1: Prepare 3 clean test tubes
Step 2: Add 1 drop of 0.1M KMnO , 5 drops of 0.1M KI, 10 drops 3% H 4
2O2 to each tube sequently
Step 3: Drip 5 drops 2M H2SO to the first two of tube and a pinch of MnO 4 to the last one 2
Step 4: Add 5 drops 3% H2O to the first two of tube 2
Step 5: Mix tubes gently, wait at least 2 minutes and observe the result 4. Reactions of KMnO4
Step 1: Add 10 drops 0.5M Na2SO into each of three test tubes. 3
Step 2: Add 5 drops 2M H2SO into the first test tube; 6M NaOH into the second test tube; and di 4 stilled water in the third test tube.
International University, Vietnam National University - HCMC 4 CHEMISTRY LABORATORY
Step 3: Add 5 drops 0.1M KMnO into each of three test tubes then observing the phenomenon. 4
5. Reactions of Fe2+ and Fe3+ Section 1: Ferric ion (Fe3+)
Step 1: Add 10 drops 0.5M FeCl into each of two test tubes. 3
Step 2: Add 5 drops 2M KOH into the first test tube and 5 drops 2M NH OH 4 into the second test tube. Then observe the phenomenon. Section 2: Ferrous ion (Fe2+)
Step 1: Add 10 drops 0.5M FeSO into each of two test tubes. 4
Step 2: Add 5 drops 2M KOH into the first test tube and 5 drops 2M NH OH 4 into the second test tube. Then observe the phenomenon. 6. Reactions of Al3+
Step 1: Add 10 drops 0.5M Al2(SO4) into each of two test tubes. 3
Step 2: Add 5 drops 2M NaOH into each of two test tubes.
Step 3: Observe the phenomenon.
Step 4: Add 20 drops 2M HCl into the first test tube and 20 drops 2M NaOH into the second test tube.
Step 5: Mix tubes gently and observe the phenomenon. 7. Flame tests
Step 1: Light the Bunsen burner.
Step 2: Clean the loop with distilled water.
Step 3: Dip the loop into the tested solution.
Step 4: Then hold it in the flame.
Step 5: Observe the color of the flame and record.
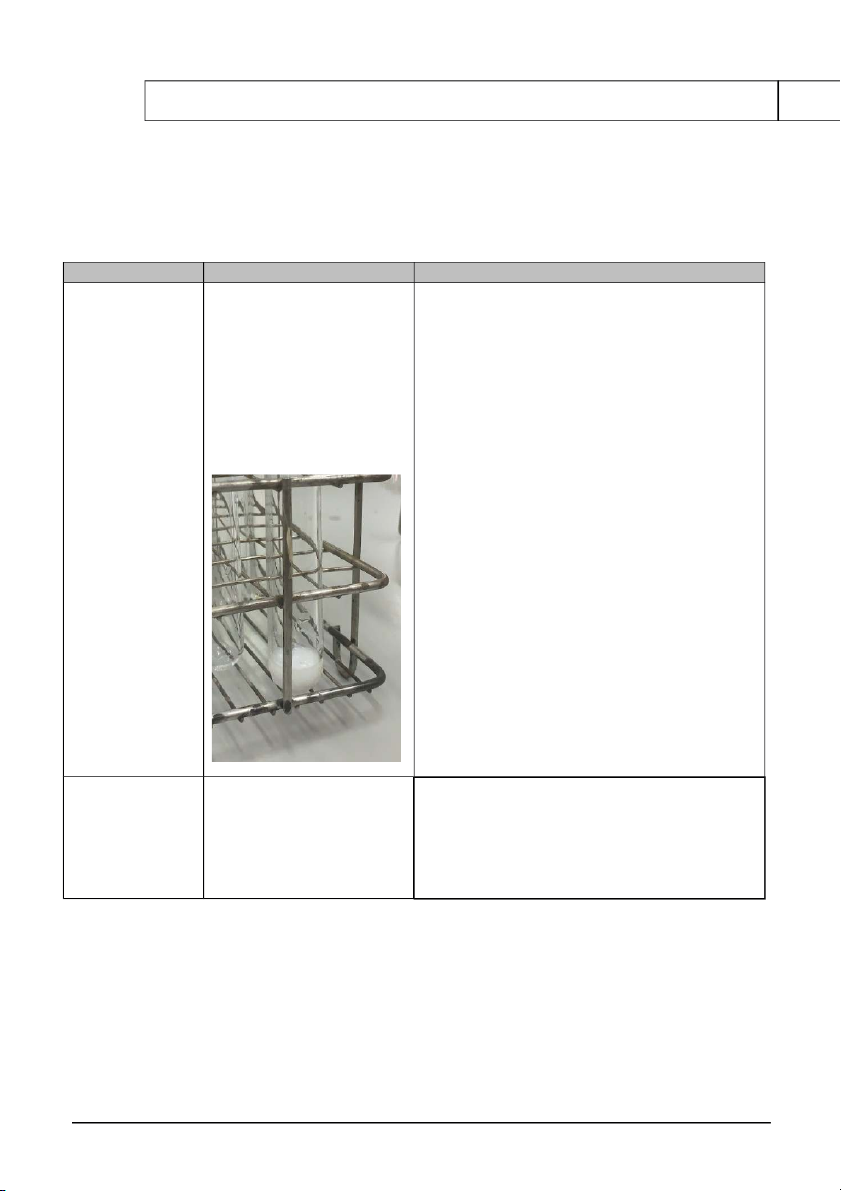
III. Results and Discussion 1. REACTIONS OF Cu2+ Reaction Observation Chemical Equation 0.5M CuSO
The formation of blue CuSO + 2NaOH → Cu(OH) 4 (↓) + Na 2 2SO (1) 4 4
International University, Vietnam National University - HCMC 5 CHEMISTRY LABORATORY + 2M NaOH precipitate 0.5M CuSO4
The formation of blue CuSO4 + 2NaOH → Cu(OH) (↓) + Na 2 SO 2 4 (1) + 2M NH4OH
precipitate but then dissolved Cu(OH)2 (↓) + 4NH OH → 4 4H O 2 + [Cu(NH3) ] 4
to form a deep blue solution (OH)2 (2) Discussion:
(1) A blue precipitate Cu(OH) 2is formed. When adding NaOH into the tube containing CuSO4, a displacement reaction occurs.
(2) A blue precipitate Cu(OH)2 appears,
then dissolves completely to form a dark blue solution
because NH4OH solution has the ability to dissolve hydroxides or slightly soluble salts of some metals
(Ag, Cu, Zn), forming complex solutions matter. These are double displacement reactions.
International University, Vietnam National University - HCMC 6 CHEMISTRY LABORATORY
2. REACTIONS OF SILVER HALIDES Reaction Observation Chemical Equation 0.5M KCl
The formation of white KCl + AgNO → KNO 3 + 3 AgCl (↓) (1) + 0.1M AgNO3 precipitate 0.5M KCl
The formation of white KCl + AgNO → KNO 3 + 3 AgCl (↓) (1) + 0.1M AgNO 3
precipitate but then AgCl (↓) + 2NH OH 4 → [Ag(NH3) ]Cl 2 + 2H O 2 + 2M NH4OH dissolved partly (2)
International University, Vietnam National University - HCMC 7 CHEMISTRY LABORATORY 0.5M KBr
The formation of light KBr + AgNO → KNO 3 3 + AgBr (↓) (3) + 0.1M AgNO3 yellow precipitate 0.5M KBr
The formation of light KBr + AgNO → KNO 3 3 + AgBr (↓) (3) + 0.1M AgNO 3
yellow precipitate but then AgBr (↓) + 2NH OH 4 → [Ag(NH3) ]Br 2 + 2H O 2 + 2M NH4OH dissolved partly (4)
International University, Vietnam National University - HCMC 8 CHEMISTRY LABORATORY Discussion:
(1) A white precipitate AgCl appears because KCl participates in the displacement reaction with AgNO3
(2) The white precipitate AgCl gradually dissolves because it reacts with NH OH 4 in a displacement
reaction to form a complex [Ag(NH3) ]Cl, 2
but does not dissolve completely due to not taking the right
amount of solution at the beginning.
(3) A light yellow precipitate AgBr appears because KBr participates in the displacement reaction
with AgNO , but then turns blac 3
k because AgBr is easily decomposed in the presence of light.
(4) The precipitate AgBr gradually dissolves because it reacts with NH OH 4 in a displacement
reaction to form a complex [Ag(NH3) ]Br 2
, but does not dissolve completely due to not taking the right
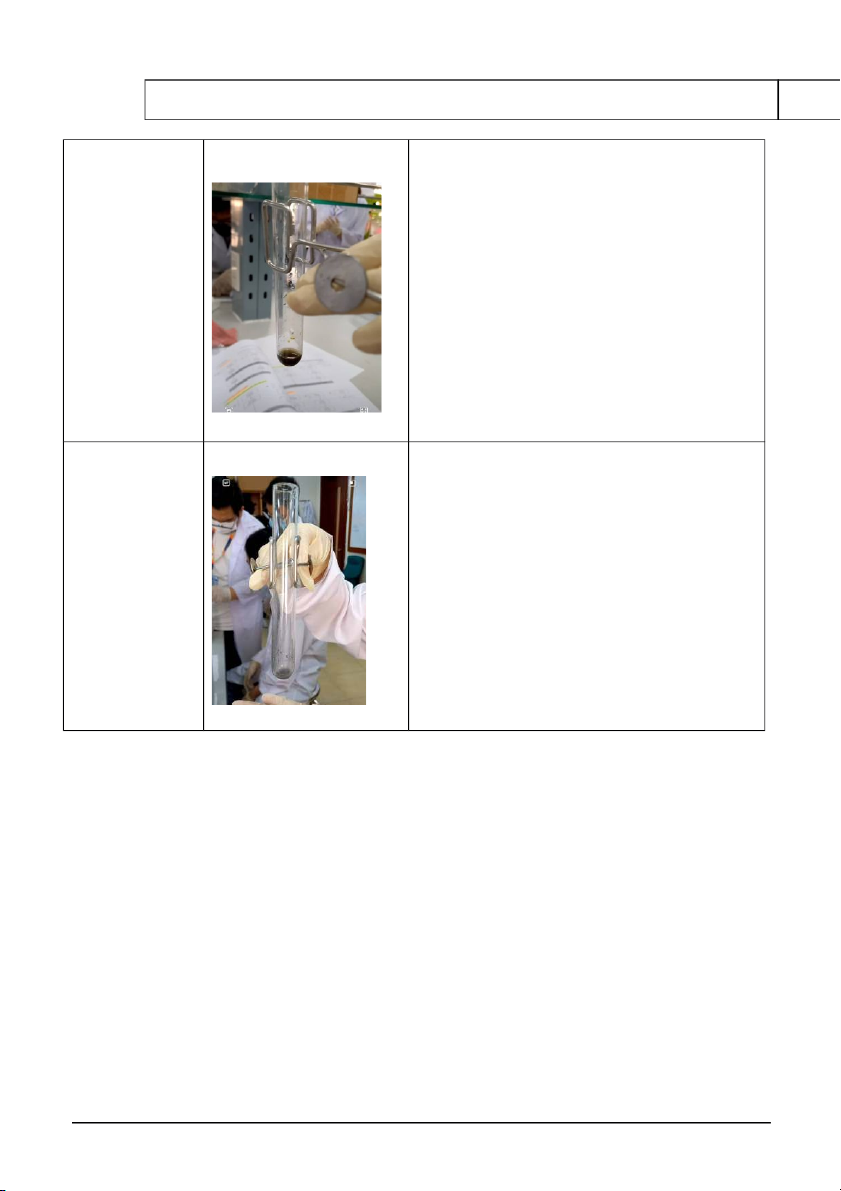
amount of solution at the beginning. 3. REACTIONS OF H2O2 Reaction Observation Chemical Equation 0.1M KMnO4
The solution becomes 2KMnO4+ 3H SO 2 4+ 5H2O2 → 5O (↑)+8H 2 2O + + 2M H2SO4 transparent 2MnSO4 + K SO 2 4 + H2O2 0.1M KI
The formation of green H2O2+ 2 KI+ H2SO4→ I2 (↓)+ K2SO4 + 2 H2O
International University, Vietnam National University - HCMC 9 CHEMISTRY LABORATORY + 2M H2SO4 precipitate + H2O2 H White gases come out 2O2
H2O2 + MnO2 → H2O + O2 (↑)+ MnO + MnO2 Discussion:
(1) KMnO 4 solution in the test tube is purple, after dropping H2SO 4 drop by drop into KMnO4
solution, no reaction occurs. Continue to add H2O2, the color of the mixture gradually fades and becomes a colorless solution.
(2) KI in the test tube is a colorless solution, the solution does not react with H2SO , a 4 few drops of
H2O2 into the mixture and the solution turns light yellow and gradually appears green precipitate.
(3) Add a small pinch of MnO 2to the test tube containing H2O ,2 with white and heat gas, MnO2 insoluble in MnO .2
-All three reactions in three tubes are redox reaction:
+ (1), KMnO4 is oxidant , H2O is reductant. 2
+ (2) , KI is a reducing agent, H2O is an oxidizing agent. 2
+ (3) , MnO is an oxidizing agent, H 2 2O is a reducing agent. 2
International University, Vietnam National University - HCMC 10 CHEMISTRY LABORATORY
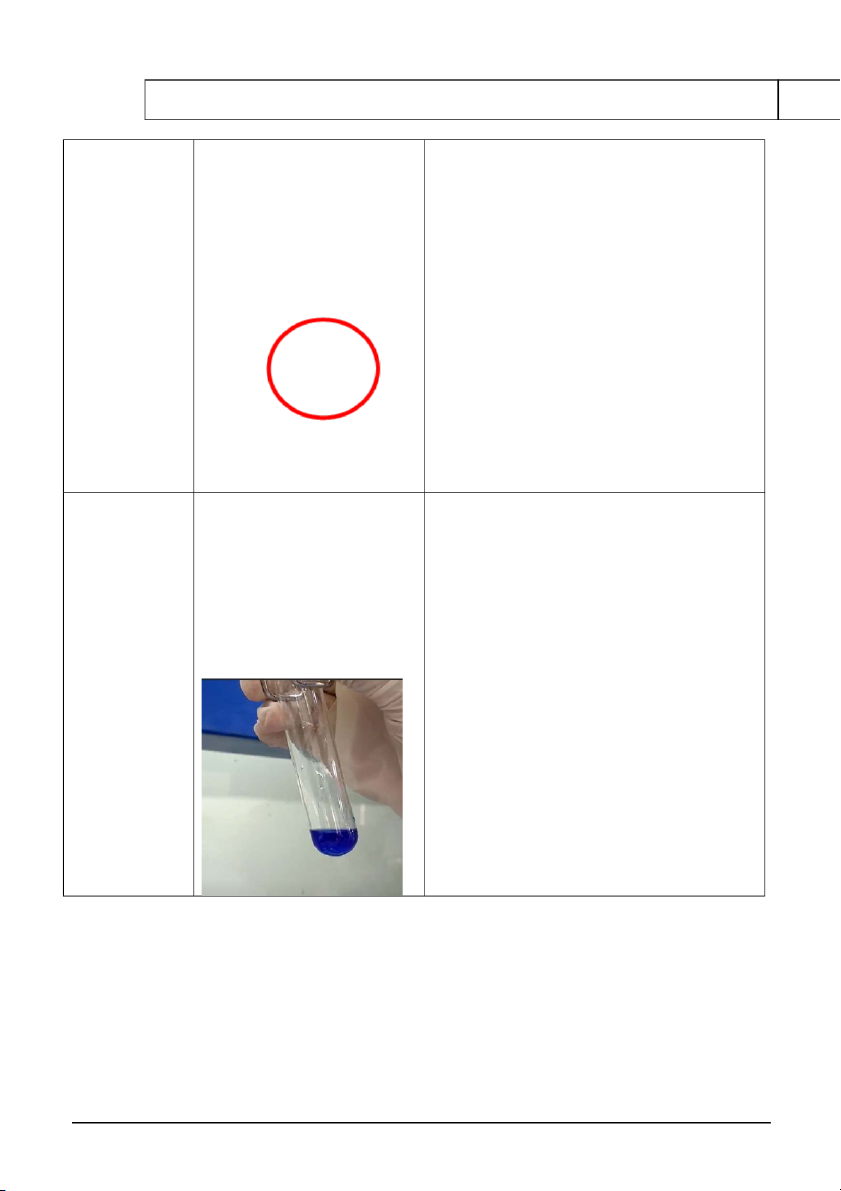
4. REACTIONS OF KMnO4 Reaction Observation Chemical Equation 0.5M Na2SO3 At first, a purple ring 5Na SO 2 3 + 3H SO 2 4 + 2KMnO 4 → K SO 2 4 + + 2M H2SO4
appears at the surface of the 5Na SO 2 4 + 2MnSO + 3H 4 O 2 + 0.1M KMnO4 solution. Then, it dissolves to transparent.




