








Preview text:
EXPERIMENT 4: CHEMICAL EQUILIBRIUM By Group 2 For Dr. Doan Hoai Linh
Course: Chemistry Laboratory Group 2 Group member.
Nguyễn Đỗ Hoài Phương BEBEIU22240
Phạm Thành Nguyên ITITDK22136
Phạm Minh Thành IELSIU22294 Tôn Duy Thành ITCSIU21234 1 TABLE OF CONTENTS
Introduction......................................................................................................................................5
Theory…………………………………………………..........................................................5
Objective…..............................................................................................................................5
Equipment and Chemical..............................................................................................................5
Procedures………………............................................................................................................5
Results and Discussion…...............................................................................................................7
Conclusion.....................................................................................................................................11 2 LIST OF FIGURE
Figure 1. Color change of K2CrO4 solution when acid and base are added………………………7
Figure 2. Color change of Methyl Violet solution when acid and base are added…………………..8
Figure 3. The observed results were as follows: (A) the reaction between 5mL of 0.1M CaCl and 2 1mL of 0.1M Na2C2O ,
4 (B) the reaction between 5mL of 0.1M CaCl 2 and 1mL of 0.1M H2C2O , 4
(C) 10 drops of 6M HCl were added and (D) 10 drops of 6M NH4OH were added to create the
result……………………………………………………………………………………………….9
Figure 4. 3mL of 0.1M CoCl2 was
combined with concentrated HCl drop-by-drop at room
temperature (A). The mixture of 3mL of 0.1M CoCl
2 and concentrated HCl was (B) heated in
warm (hot water), (C) cooled in a cool (ice bath). The mixture of 3mL of 0.1M CoCl 2 and
concentrated HCl in another tube was (D) cooled in a cool (ice bath), (E) heated in warm (hot
water)…………………………………………………………………………………………….11 LIST OF TABLE Table 1. Acid/Base
Equilibria……………………………………………………………………...7 Table 2. Equilibria of Acid/Base
Indicators……………………………………………………......8
Table 3. Equilibria of precipitation reactions……………………………………………………...9
Table 4. Temperature effects on equilibria……………………………………………………….10 3 Assignments Number Full name Student ID Contribution 1
Nguyễn Đỗ Hoài Phương BEBEIU22240 100% 2 Tôn Duy Thành ITCSIU21234 100% 3 Phạm Thành Nguyên ITITDK22136 100% 4 Phạm Minh Thành IELSIU22294 100% 4 I. Introduction 1.1 Theory
Chemical equilibrium is a state reached by the reaction mixture when the rate of forward
and reverse reaction is equal.
At equilibrium: Rateforward = Ratereverse
The equilibrium system will adjust itself according to Le Chatelier’s Principle when
having some stresses on the reaction.
Le Chatelier’s Principle: When a system in equilibrium is disturbed by a change of
temperature, pressure or concentration, the reaction shifts in equilibrium composition in a way
that tends to counteract this change of variables. 1.2 Objective
How to observe the effect of applying stresses on chemical systems at equilibrium
How to apply Le Chatelier’s Principle to explain the changes in the system II. Experiment methods 2. Procedure
Equipments: Preparing the necessary equipment for conducting an experiment: Erlenmeyer
flask, beaker, cylinder, water bath.
Chemicals: Experiments need to prepare the following chemicals: distilled water, kali chromat (K CrO 2 ), 4
concentrated HCl, natri hydroxide (NaOH), methyl violet, calcium chloride (CaCl ), 2 natri oxalat (Na2C2O ), axi 4 t oxalic (H2C2O ), amoni 4 hydroxide (NH OH), carbon 4 dichloride oxide (CoCl ). 2
2.1 Acid/ Base equilibria
Equilibrium system: 2CrO 2- + 2- 4
(aq) + 2H (aq) ⇌ Cr2O7 (aq) 2 + H O (l)
For this experiment, we used three separate tubes. To begin with, we added 10 drops of 0.5M K Cr 2 2O to each tube. 4
In the first tube, we observed any changes in color. Next, we added 5
drops of concentrated HCl to the second tube and noted the resulting color change, comparing it
with the first tube. We followed the same process for the third tube, adding 5 drops of
concentrated HCl initially and comparing the color change with the first tube. After that, we 5
added an additional 10 drops of 6M NaOH to the third tube and observed any resulting color
change, comparing it with the second tube.
2.2 Equilibrium of acid/ base indicators
Equilibrium system: H(MV) - (aq) + H O 2
(l) ⇌ H3O+ (aq) + MV (aq)
In the second part of the experiment, two drops of methyl violet are combined with 20ml
of distilled water. This mixture is then evenly distributed into two separate tubes. In the first tube,
the color change is observed. In the second tube, 6M HCl is added incrementally until no further
color change is observed at this point, the color change is noted. Subsequently, 6M NaOH is
added until no additional color changes occur, and again, the color change is recorded. Finally,
more 6M HCl is introduced until there is no further color alteration, and once more, the final color change is observed.
2.3 Equilibria of precipitation reactions
Equilibrium system: Ca2+ 2-
(aq) + C2O4 (aq) ⇌ CaC2O4 (s)
In the third experiment, two tubes are prepared, each containing 5ml of 0.1M CaCl2
solution. To the first tube, 1ml of 0.1M Na2C2O
4 is added, and color change is observed. In the
second tube, 1ml of 0.1M H2C2O
4 is added, and the color change is noted for comparison with
the first tube. Subsequently, 10 drops of 6M HCl are added to both tubes, and any resulting color
changes are observed. Finally, 10 drops of 6M NH OH 4
are added to each tube, and any further color changes are recorded.
2.4 Temperature effects on equilibria
Equilibrium system: [Co(H -
2O)6]2+ (aq) + 4Cl (aq) ⇌ (CoCl4)2- (aq) + 6H2O(l)
In the fourth experiment, prepare a solution of 3ml of 0.1M CoCl2 and slowly add
concentrated HCl until a purple-violet color is observed, repeating the addition if a deep blue
color appears. Subsequently, divide the resulting solution evenly into three separate tubes for
further observation. The first tube is to be kept at room temperature to monitor any color
changes. The second tube should be heated with warm water and then cooled using an ice bath,
with color observations made after each step for comparison with the first tube. The third tube is
initially cooled with an ice bath, followed by heating using a hot water bath, and color changes
after each step are noted and compared with the first tube.
IV. Result and discussion 6 1. Acid/Base Equilibria
Table 1. Acid/Base Equilibria.
Equilibrium System: 2CrO 2- + 2- 4 (aq) + 2H (aq) Cr ⇌ 2O7 + H (aq) O 2 (l) Addition Predicted outcome Observation Initial solution Yellow Yellow + Concentrated HCl Orange Orange + 6M NaOH Yellow Yellow
In table 1, the presence of the chromate ion (CrO 2- 4
) resulted in the solution appearing
yellow as shown in figure 1(A).
Adding hydrochloric acid (HCl) boosted the concentration of hydrogen ions (H+). This
triggered a reaction between chromate ions (CrO4²⁻ ), originally lending the solution a yellow
color, and H+, forming dichromate ions (Cr 2-
2O7 ) that are orange. To compensate for the rise in
H+, the equilibrium favored the production of more dichromate ions, causing the solution to turn
orange from yellow as shown in figure 1(B).
Adding sodium hydroxide (NaOH) after hydrochloric acid (HCl) increased the
concentration of hydroxide ions (OH⁻ ). This caused OH⁻ to react with the previously formed dichromate ions (Cr O 2- 2
7 ), which are orange. The reaction consumes H⁺ ions, lowering their
concentration. As a response, the equilibrium shifts back towards chromate ions (CrO4²⁻ ), which
are yellow, causing the solution's color to return from orange to yellow as in figure 1(C). 7(A) (B) (C)
Figure 1. The initial K CrO 2 4 solution (A), the K CrO 2
4 solution when added concentrated HCl
(B), the K2CrO4 solution when added 6M NaOH (C).
2. Equilibria of Acid/Base Indicators
Table 2. Equilibria of Acid/Base Indicators
Equilibrium System: H(MV) + (aq) + H2O(l) H ⇌ O 3 (aq) + MV-(aq) Addition Predicted outcome Observation None Purple Purple (control) +6M HCl Yellow Green Blue +6M NaOH Purple Purple +6M HCl Blue Blue
In table 2, dissolving methyl violet in distilled water creates a bright purple solution. This
color is due to the inherent color properties of methyl violet, rather than any chemical reaction
that occurs when it mixes with water as shown in Figure 2(A).
Adding hydrochloric acid (HCl) to the solution increases the concentration of hydrogen
ions (H+). This causes the pH to decrease, and the methyl violet indicator changes color from a
vibrant purple to a greenish-yellow. This color change signifies a shift in the equilibrium towards
the acidic form of the indicator. In the above experiment, it is possible that adding H+ is not
enough to cause methyl violet to not change to yellow-green but instead change to blue as shown in Figure 2(B).
Introducing sodium hydroxide (NaOH) to the solution mops up hydrogen ions (H+). This reaction, H+ + OH- → H O, 2
drives the pH upwards. As a consequence, the color of the methyl
violet indicator reverts to purple. This shift signifies a change in equilibrium towards the basic
form of the indicator, which alters the molecule's structure and results in a brighter purple color
for the solution as shown in Figure 2(C). 8 (A) (B) (C) (D)
Figure 2. The initial methyl violet solution (A), the methyl violet solution when added 6M HCL
(B), the methyl violet solution added 6M NaOH (C), the solution when added 6M HCL (D).
Adding more hydrochloric acid (HCl) to the solution bumps up the concentration of hydrogen
ions (H+). This dip in pH makes the methyl violet indicator switch colors, going from a beautiful
blue-violet to a more blue-green hue. This color change reflects a shift in equilibrium back
towards the acidic form of the indicator as shown in Figure 2(D).
3. Equilibria of precipitation reactions.
Table 3: Equilibria of precipitation reactions.
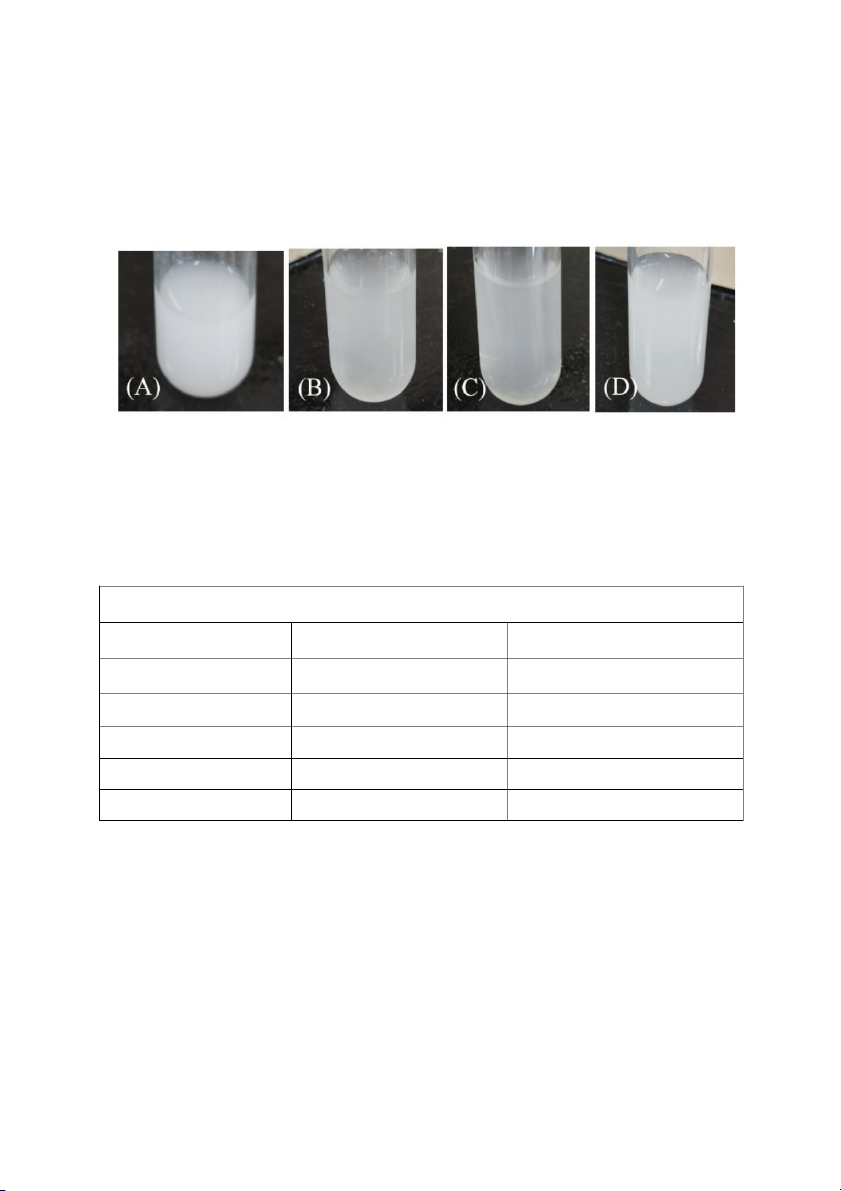
Equilibrium System: Ca2+ 2- (aq) + C2O4 (aq) CaC ⇌ O 2 4 (s) Addition Predicted outcome Observation 10mL 0.1M Na2C2O White White 1mL 0.1M H2C O 2 4 White White 10 drops 6M HCl White White 10 drops 6M NH4OH White White
In table 3, the addition of 5mL of 0.1M CaCl
2 to a test tube followed by the addition of 1mL of 0.1M Na2C O 2
4 resulted in the formation of a white precipitate. This suggested the formation of CaC O 2 ,
4 which was insoluble in water. The appearance of a white precipitate
indicated a chemical reaction between calcium ions (Ca2+) from CaCl 2- 2 and oxalate ions (C O 2 4 ) from Na2C O 2 shown in Figure 3(A). 4
The addition of 5mL of 0.1M CaCl to a test tube 2
followed by the addition of 1mL of 0.1M
H2C2O4 resulted in the formation of a white precipitate, like the observation in the previous
experiment. This suggested that calcium oxalate precipitated out of the solution again, indicating
a reaction between calcium ions from CaCl2 and oxalate ions from H2C O 2 , forming the 4 insoluble CaC2O4 shown in Figure 3(B). 9
Upon adding 10 drops of 6M HCl, the white precipitate remained the same but somewhat
lighter, which was also due to the reaction of 6M HCl with CaC O 2 4 to form H2C2O4 white crystals shown in Figure 3(C).
Finally, adding 10 drops of 6M NH OH to the mixture in 4
the test tube, it was observed that
the solution was whiter than before, due to the reaction to form (NH4)2C O 2 4 precipitate shown in Figure 3(D).
Figure 3. The observed results were as follows: (A). the reaction between 5mL of 0.1M CaCl2
and 1mL of 0.1M Na2C2O4, (B). the reaction between 5mL of 0.1M CaCl2 and 1mL of 0.1M H2C2O ,
4 (C). 10 drops of 6M HCl were added and (D). 10 drops of 6M NH OH 4 were added to create the result.
4. Temperature effects on equilibria.
Table 4: Temperature effects on equilibria.
Equilibrium System: [Co(H O) − 2 6]2+ (aq) + 4Cl (aq) (CoCl ⇌ 4)2− (aq) + 6H2O (l) Addition Predicted outcome Observation Room temperature Purple-violet Light purple-violet Warm (hot water) Blue Dark blue-violet Cool (ice bath) Red Light red-pink Cool (ice bath) Red Red-pink Warm (hot water) Blue Dark purple-violet
In table 4, when 3mL of 0.1M CoCl2 was added
to the test tube and concentrated HCl was
added drop by drop, the solution was stopped when it turned purple-violet. The change in color
to purple-violet indicated the formation of the complex ion [Co(H2O)6]2+ due to the coordination
of chloride ions from the HCl with cobalt ions from CoCl shown in Figure 4(A). 2
Upon heating the test tube containing the solution, the color changed from purple-violet to
dark blue-violet. The observed change in color from purple-violet to dark blue-violet upon 10
heating suggested further formation or stabilization of the complex [CoCl4]2- due to the increased
temperature shown in Figure 4(B).
Finally, changing the environment of the test tube from warm (hot water) to cool (ice bath)
caused the color of the solution to change from dark blue-violet to light red-pink shown in Figure 4(C).
After taking the tube to a cool ice bath, the solution's color changed to red-pink. The
change in color from purple-violet to red-pink upon cooling suggested the formation of the hydrated complex ion [CoCl (H 4 O) 2
2]2-, indicating a shift in the equilibrium towards the hydrated
form of the cobalt complex shown in Figure 4(D).
Upon heating the test tube in warm (hot water), the solution reverted to its initial purple-
violet color but somewhat darker shown in Figure 4(E).
Figure 4. (A). 3mL of 0.1M CoCl2 was combined with concentrated HCl drop-by-drop at room
temperature. The mixture of 3mL of 0.1M CoCl
2 and concentrated HCl was (B). heated in warm
(hot water), (C). cooled in a cool (ice bath). The mixture of 3mL of 0.1M CoCl and concentrated 2
HCl in another tube was (D). cooled in a cool (ice bath), (E). heated in warm (hot water). V. Conclusion
To sum up, after carrying out experiment 4, which are gained a better understanding of
reversible reactions and recognizing the effects of different types of stresses upon reactions at
equilibrium point as well as determining the cause of changes in the system by Le Chatelier's
Principle. With experiences from prior experiments, the preparation in using equipments are
more professional and careful, following procedures and safety regulations, distributing
workload evenly and performing different reactions correctly which resulted in finishing
experiment 4 smoothly and on time. 11




