













Preview text:
lOMoARcPSD|364 906 32
International University, Vietnam National University - HCMC1 CHEMISTRY LABORATORY REPORT
EXPERIMENT 1: CHEMICAL REACTIONS
Group: ______________ Class: ______________ Date: ____________ Group members: Full name Student ID Declaration of Contribution Signature 1 2 3 4 5 Total score: _______/100 Part 1. Introduction
(Introduce the general background and summarize the aims/objectives of the experiment)
Part 2. Materials and Methods
(Summarize the experimental design/structure of your experiment)
Part 3. Results and Discussion
1 . REACTIONS OF Cu 2+ Reaction Observation Chemical Equation 0. M
5 CuSO 4 + 2M NaOH
0.5 M CuSO 4
+ 2M NH 4 OH Discussion: lOMoARcPSD|364 906 32
International University, Vietnam National University - HCMC2 CHEMISTRY LABORATORY
(Discuss: i) Explanation for the above observation; ii) Possible reasons for any misconducted results)
2. REACTIONS OF SILVER HALIDES Reaction Observation Chemical Equation 0.5 M KCl
+ 0.1M AgNO 3 0.5 M KCl + 0.1M AgNO 3
+ 2M NH 4 OH 0.5 M KBr
+ 0.1M AgNO 3 0.5 M KBr
+ 0.1M AgNO 3
+ 2M NH 4 OH Discussion:
3 . REACTIONS OF H 2 O 2 Reaction Observation Chemical Equation
0.1 M KMnO 4
+ 2M H 2 SO 4
+ H 2 O 2 0. M 1 KI
+ 2M H 2 SO 4
+ H 2 O 2
H 2 O 2 + MnO 2 Discussion: lOMoARcPSD|364 906 32
International University, Vietnam National University - HCMC3 CHEMISTRY LABORATORY
4 . REACTIONS OF KMnO 4 Reaction Observation Chemical Equation
0.5 M Na 2 SO 3
+ 2M H 2 SO 4
+ 0.1M KMnO 4
0.5 M Na 2 SO 3 + 6N NaOH
+ 0.1M KMnO 4 0. M
5 Na 2 SO 3
+ H 2 O
+ 0.1M KMnO 4 Discussion: .
5 A. REACTIONS OF Fe 3+ Reaction Observation Chemical Equation
0.5 M FeCl 3 + 2M KOH
0.5 M FeCl 3
+ 2M NH 4 OH Discussion:
5 . B. REACTIONS OF Fe 2+ Reaction Observation Chemical Equation
0.5 M FeSO 4 + 2M KOH
0.5 M FeSO 4
+ 2M NH 4 OH Discussion: lOMoARcPSD|364 906 32
International University, Vietnam National University - HCMC4 CHEMISTRY LABORATORY .
6 REACTIONS OF Al 3+ Reaction Observation Chemical Equation
0.5 M Al 2 ( SO 4 ) 3 + 2N NaOH + 2M HCl
0.5 M Al 2 ( SO 4 ) 3 + 2M NaOH + 2M NaOH Discussion: . 7 FLAME TEST Solution Dominant flame Wavelength Frequency Photon energy (J) color ( nm )
( s 1 ) LiCl NaCl KCl CaCl 2 BaCl 2 Discussion : Part 4. Conclusions END. lOMoARcPSD|364 906 32
International University, Vietnam National University - HCMC5 CHEMISTRY LABORATORY REPORT
EXPERIMENT 2: pH AND BUFFERS
Group: ______________ Class: ______________ Date: ____________ Group members: Full name Student ID Declaration of Contribution Signature 1 2 3 4 5 Total score: _______/100 Part 1. Introduction
(Introduce the general background and summarize the aims/objectives of the experiment)
Part 2. Materials and Methods
(Summarize the experimental design/structure of your experiment)
Part 3. Results and Discussion lOMoARcPSD|364 906 32
International University, Vietnam National University - HCMC6 CHEMISTRY LABORATORY .
1 pH OF DEIONIZED WATER Observed pH Time 1 st 2 nd Discussion
( second ) ( Group___ ) ( Group__ ) _ 0 20 40 60
2 . pH OF STRONG ACID Measured pH Theoretical Solution Discussion 1 st 2 nd pH ( Group___ ) ( Group____ )
10 mL of 0.1M HCl Add 90 mL of distilled water Add 10 mL of 0.1 M NaOH Add 90 mL of 0.01 M NaOH Calculation: 3. pH OF WEAK ACID Solution Measured pH K a Discussion 1 st 2 nd ( Group___ ) ( Group____ )
0.1 M acetic acid
0.01 M acetic acid
0.001 M acetic acid Calculation: lOMoARcPSD|364 906 32
International University, Vietnam National University - HCMC7 CHEMISTRY LABORATORY . 4 pH OF SALTS Measured pH Predicted Solution 1 st 2 nd Discussion pH ( Group____ ) ( Group____ ) 0.1 M NaCl
0.1 M CH 3 COONa
0.1 M NH 4 Cl Calculation: .
5 pH OF BUFFERS Volume Volume (mL) Measured pH Calculated
Buffer ( mL) 0.1M 0.1 M Acid Base 1 st 2 nd pH
CH 3 COOH
CH 3 COONa ( Group__ ) ( Group__ ) A 10.0 40.0 B 40.0 10.0
Calculation pH: Discussion:
Part I: Addition of 10 drops 0.1 M HCl Buffer pH from pH after
Total volume HCl (drops) Discussion the start, adding 10
to change pH by one unit pH o drops HCl
( pH o -1) A B
Part II: Addition of 10 drops 0.1 M NaOH lOMoARcPSD|364 906 32
International University, Vietnam National University - HCMC8 CHEMISTRY LABORATORY pH from pH after Total volume NaOH Buffer the start, adding 10
( drops) to change pH by Discussion pH o drops NaOH
one unit (pH o +1) A B Part 4. Conclusions END. REPORT
EXPERIMENT 3: REDOX TITRATION
Group: ______________ Class: ______________ Date: ____________ Group members: Full name Student ID Declaration of Contribution Signature 1 2 3 4 5 Total score: _______/100 Part 1. Introduction
(Introduce the general background and summarize the aims/objectives of the experiment) lOMoARcPSD|364 906 32
International University, Vietnam National University - HCMC9 CHEMISTRY LABORATORY
Part 2. Materials and Methods
(Summarize the experimental design/structure of your experiment)
Part 3. Results and Discussion
1. STANDARDIZATION OF PREPARED KMnO4 SOLUTION Calculation:
Normality of the standard H2C2O4 solution, N(H2C2O4) = ___________________
Volume of the standard H2C2O4 solution used, V(H2C2O4) = ___________________
Trial # Burette reading (mL) Volume of KMnO 4 (mL) Normality of KMnO 4 (N) 1 - 2 -
Average Normality of KMnO4 = ____________ Discussion:
2. DETERMINATION OF UNKNOWN CONCENTRATION H2C2O4 SOLUTION Calculation:
Normality of the standard KMnO4 solution, N(KMnO4) = ___________________
Volume of the unknown H2C2O4 solution used, V(H2C2O4) = ___________________
Trial # Burette reading (mL) Volume of KMnO 4 (mL) Normality of H 2 C 2 O 4 (N) 1 - 2 - lOMoARcPSD|364 906 32
International University, Vietnam National University - HCMC10 CHEMISTRY LABORATORY
Average Normality of H2C2O4 = ____________ Discussion:
3. DETERMINATION OF UNKNOWN CONCENTRATION FeSO4 SOLUTION Calculation:
Normality of the standard KMnO4 solution, N(KMnO4) = ___________________
Volume of the unknown FeSO4 solution used, V(FeSO4) = ___________________
Trial # Burette reading (mL) Volume of KMnO 4 (mL) Normality of FeSO 4 (N) 1 - 2 -
Average Normality of FeSO 4 = ____________ Discussion: Part 4. Conclusions END. REPORT
EXPERIMENT 4: CHEMICAL EQUILIBRIUM
Group: ______________ Class: ______________ Date: ____________ Group members: lOMoARcPSD|364 906 32
International University, Vietnam National University - HCMC11 CHEMISTRY LABORATORY Full name Student ID Declaration of Contribution Signature 1 2 3 4 5 Total score: _______/100 Part 1. Introduction
(Introduce the general background and summarize the aims/objectives of the experiment)
Part 2. Materials and Methods
(Summarize the experimental design/structure of your experiment)
Part 3. Results and Discussion .
1 ACID/BASE EQUILIBRIA
Equilibrium System: 2 CrO 4 2 + 2H + ( aq) Cr 2 O 7 2 + H 2 O(l) Description of Predicted Observation Discussion conditions outcome Initial solution + Conc. HCl + 6 N NaOH Note: lOMoARcPSD|364 906 32
International University, Vietnam National University - HCMC12 CHEMISTRY LABORATORY
2 . EQUILIBRIA OF ACID/BASE INDICATORS
Equilibrium System: H(MV)(aq) + H 2 O(l) H 3 O + ( aq) + MV (aq ) Addition Predicted Observation Discussion outcome None (control) 6 M HCl 6 M NaOH 6 M HCl Note:
3. EQUILIBRIA OF PRECIPITATION REACTIONS lOMoARcPSD|364 906 32
International University, Vietnam National University - HCMC13 CHEMISTRY LABORATORY 2+
2 4 2 ( aq)
CaC 2 O 4 ( s ) Predicted Addition Observation Discussion outcome Test tube 1: 0.1 M Na 2 C 2 O 4 Test tube 2: + 0.1 M H 2 C 2 O 4 Test tube 2: + 6 M HCl Test tube 2: + 6 M NH 4 OH Note:
Equilibrium System: Ca (aq) + C O lOMoARcPSD|364 906 32
International University, Vietnam National University - HCMC14 CHEMISTRY LABORATORY
4. TEMPERATURE EFFECTS ON EQUILIBRIA Equilibrium System: 2+ 2
Co(H 2 O) 6 ( aq) + 4Cl ( aq) CoCl 4 (aq
) + 6H 2 O(l) Description of Predicted Observation Discussion conditions outcome Nothing changed ( control ) Hot water bath Ice-water bath Note: Part 4. Conclusions END. REPORT
EXPERIMENT 5: FACTORS AFFECTING REACTION RATE
Group: ______________ Class: ______________ Date: ____________ Group members: lOMoARcPSD|364 906 32
International University, Vietnam National University - HCMC15 CHEMISTRY LABORATORY Full name Student ID Declaration of Contribution Signature 1 2 3 4 5 Total score: _______/100 Part 1. Introduction
(Introduce the general background and summarize the aims/objectives of the experiment)
Part 2. Materials and Methods
(Summarize the experimental design/structure of your experiment)
Part 3. Results and Discussion
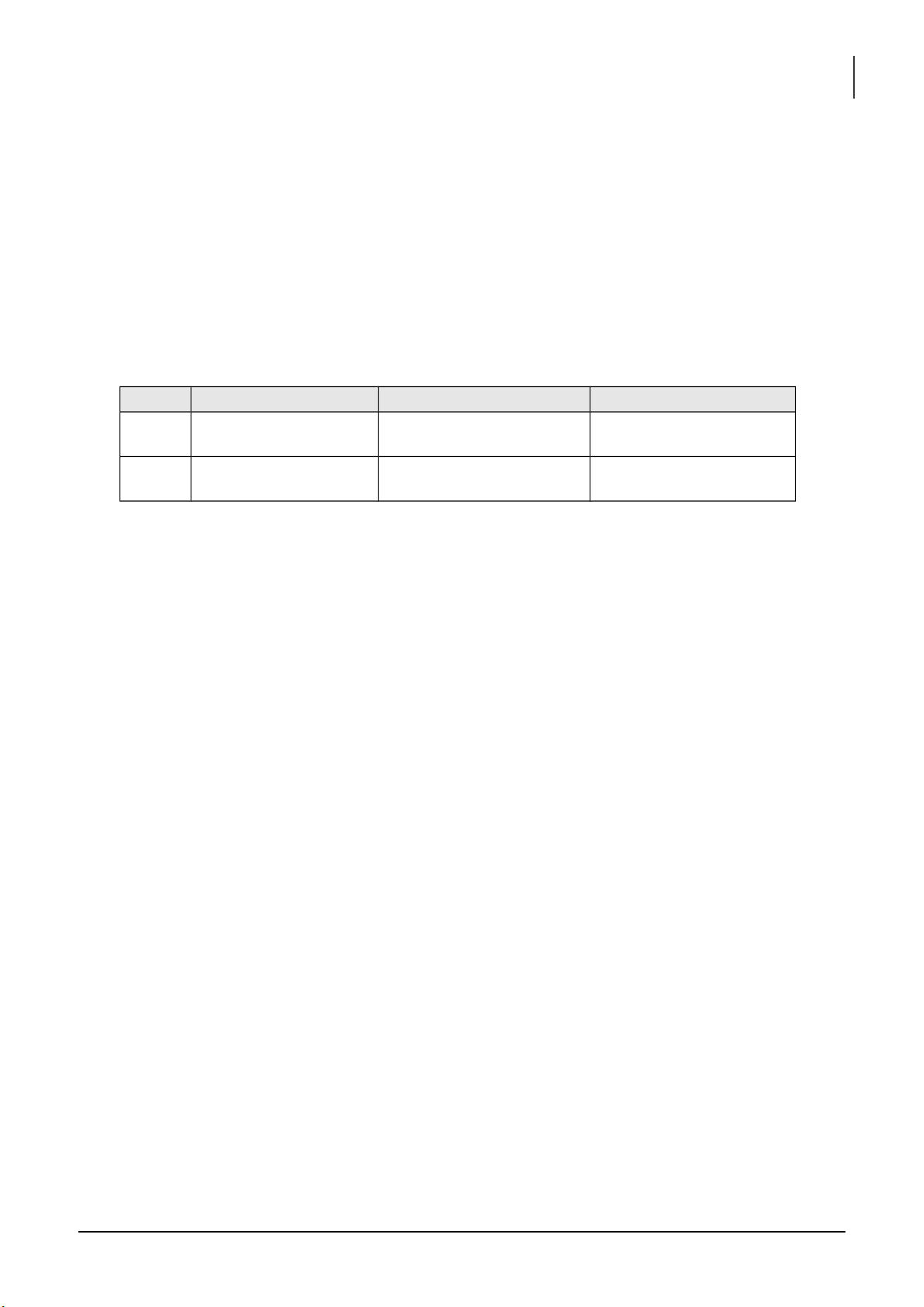
1. EFFECT OF CONCENTRATION ON REACTION TIME
Reaction 1: __________________________________________________
Reaction 2: __________________________________________________
Calculate the initial concentrations of I- and S 2- 2O8 ions: Mixture # 5: [I-] = [S2O82-] = lOMoARcPSD|364 906 32
International University, Vietnam National University - HCMC16 CHEMISTRY LABORATORY
Plotting the concentration of iodide ion versus time: [Note: X – axis: time; Y – axis: concentrations]. * Mixtures # 1-6: - Graph
- The order of reaction with respect to iodide ion? - Discussion:
* Mixtures # 1, 7, 8, 9, 10, and 11: - Graph
- The order of reaction with respect to iodide ion? lOMoARcPSD|364 906 32
International University, Vietnam National University - HCMC17 CHEMISTRY LABORATORY - Discussion:
2. EFFECT OF TEMPERATURE ON THE REACTION RATE Reaction System: Description of Predicted Reaction Observation Discussion conditions outcome time Room temperature 50 0 C 90 0 C Note:
3. EFFECT OF A CATALYST ON THE REACTION RATE Reaction System: Description of Predicted Observation Trial Discussion conditions outcome
( Reaction rate ) 1 + MnCl 2 2 + MnO 2 3 + NaCl 4 + CaCl 2 5 + Zn 6 + KNO 3 7 + Fe(NO 3 ) 3
The order of catalyst activity: Note: lOMoARcPSD|364 906 32
International University, Vietnam National University - HCMC18 CHEMISTRY LABORATORY Part 4. Conclusions END.
Document Outline
- REPORT
- EXPERIMENT 1: CHEMICAL REACTIONS
- REPORT (1)
- EXPERIMENT 2: pH AND BUFFERS
- REPORT (2)
- EXPERIMENT 3: REDOX TITRATION
- REPORT (3)
- EXPERIMENT 4: CHEMICAL EQUILIBRIUM
- REPORT (4)




