





Preview text:
VIETNAM NATIONAL UNIVERSITY HO CHI MINH CITY
INTERNATIONAL UNIVERSITY CHEMICAL ENGINEERING
Carriers: A Report - Laboratory
Experiment 3: THIN LAYER CHROMATOGRAPHY
Instructor: Dr. Le Quang Phong
Date of submission: 1/11/2023 Group 03
Student’s full name
Student’s ID Nguyễn Sỹ Khôi BTCEIU21092 Lê Gia Khương BTCEIU21093 Nguyễn Tiến Phúc BTCEIU21112 Đinh Nguyễn Quốc Thắng BTCEIU21115 Contents
I. ABSTRACT ............................................................................................................................... 3
II. INTRODUCTION ................................................................................................................... 3
III. MATERIALS AND METHODS ........................................................................................... 4
Materials: .................................................................................................................................... 4
Methods: ...................................................................................................................................... 4
IV. RESULT AND DISCUSSION ................................................................................................ 5
Result: ........................................................................................................................................ 5
Discussion: ................................................................................................................................ 7
V. CONCLUSION .......................................................................................................................... 8
VI. REFERENCES ........................................................................................................................ 8 2 I. ABSTRACT
The process of Thin Layer Chromatography allows compounds to rise on a TLC plate
through capillary action as the chromatogram develops. Depending on the polarity of the solvent
and how tightly bonded the compounds are to the stationary phase figure out how far the compound
will rise the TLC plate. Using the TLC plates, the Rf values were calculated for each of the solutions.
With these Rf values, the unknown sample was identified by comparing the other Rf values. The
discussion encompasses the several types of stationary phase, including silica gel, alumina, and
cellulose, and their respective applications in different chemical systems. Chromatography is based
on the principle where molecules in a mixture are applied onto the surface or into the solid, and
fluid stationary phase. TLC was performed on a sheet of an inert substrate such as glass, plastic,
or aluminum foil, which was coated with a thin layer of adsorbent material, usually silica gel,
aluminum oxide, or cellulose. The mixture to be separated was spotted on the sheet and placed in
a solvent, which traveled up the sheet by capillary action and separated the components of the
mixture. The silica gel was used for TLC because it was the most used adsorbent by far and
continues to be the dominant stationary phase for TLC. For the chromatography of important
compounds, the surface of silica gel with the highest concentration of geminal and related silanols
was most preferred because these silanols were less acidic.
When the solvents had risen to near the top of the plate, the plate was removed, dried, and
visualized using UV light. Variations on this protocol were used for different purposes, including
pretreating the sample, changing the sorbent, plate material, the solvent, system, the development
techniques, and method of detection and visualization or by coupling TLC to other techniques. In
general, TLC could be coupled with other techniques, such as mass spectrometry or infrared
spectroscopy, for further identification or characterization of the components. II. INTRODUCTION
Thin Layer Chromatography was an analytical technique that was simple and inexpensive.
TLC helped one determine the number of compounds in a mixture. The TLC allowed one to
determine whether compounds were identical. In TLC plates were coated with a thin layer of
absorbent that served as the stationary phase, while the solvent is a mobile phase that pushed
compounds up the TLC plate. The solvent rose the plate via capillary action allowing the 3
chromatogram to develop. The distance and speed travelled by solvents up the chromatogram
depended on how tightly bonded the compound was to the stationary phase. The polarity of the
solvent also determined how far a solvent would travel up the TLC plate. Once the solvent front
was about a centimeter from the top of the plate it had to be marked for the Rf value to be calculated.
This experiment introduced the mechanics of TLC, and the chemical principles behind it.
In the first part, separate the soluble components of spinach extract, second part was analyzing the
compounds and separated by extraction in the last lab. The ratio of the distance a compound moved
to the distance the solvent moved was Rf value (retention factor). This value was characteristic of
the compound, the solvent, and the stationary phase, and the formula was:
distance traveled by substances Rf =
dis tan ce traveled by solvent front III. MATERIALS AND METHODS Materials: • 3 Vials • UV Champer • Aluminum foil • Silica plate for TLC • Pipets
• Boric acid and Oxalic acid • Cylinder 5 mL • Iodine • Alcohol lamp • Methanol • Spatula • Hexane • Forceps • Distilled water • Beaker 100 mL • Acetone • Capillary Methods:
The methods of this report have 3 separate parts: preparation of spinach leaf extract,
analytical Chromatography of spinach, and observation and detection of phytocomponents. First,
in the preparation part, get about 5g of a spinach leaf and tear it into small pieces (about 3-5 mm).
After that, put it in the mortar with 10 ml methanol then grind the mixture slowly and firmly till
the solvent has a dark green color. Next, the liquid extracted continues to transfer into the given
glass vial (carefully remove the ground leaves and stems), and then heat the glass vial with the 4
solution to condense the extract about 1/5 of the initial volume. Moving to the next step, add a
small portion of Hexane to the sample (about 3 times and 5ml for each, until the sample is
converted to colorless). In the analytical chromatography of spinach, prepare 10 ml of developing
solvent (70:30 hexane-acetone) in the beaker. After that, get a TLC plate for development then
draw a faint pencil around 1cm from the bottom edge and one round 0.5 from the top for the solvent
front. Then use the liquid extract in all parts to make a small spot on the plate about 5-10 times
(spots should be air dry thoroughly, equal in size) and put the TLC plate into the beaker until the
solvent reaches the solvent front (the TLC plate should not touch the edge of the beakers). Finally,
in the observation and detection of phytocomponents, there are 3 ways. First, look at the TLC plate
under the room light and in UV chambers. Secondly, spray the plate with a mixture of 15ml 3%
boric acid solution and 5ml 10% oxalic acid, then heat the plate and record the result. The final
step is to place the TLC plate in iodine-containing chambers and record the observations. IV. RESULT AND DISCUSSION • Result:
After preparing a solvent mixture consisting of 70% hexane and 30% acetone, the thinlayer
chromatography (TLC) plate was subjected to heat until complete evaporation of the solvent. It
could be observed that sample A had three, sample B had two, sample C had one, sample D had
two, and sample E had three organic compounds.
Figure 1: TLC plate observation after dipping in 70:30 hexane-acetone.
Then, the TLC plate was stimulated by UV light to visibly two dots at sample C and D. 5
Figure 2: TLC plate observation under UV light.
Figure 3: TLC plate after stimulated by UV light.
Finally, placed the TLC plate in beaker with Iodine and covered with aluminum foil to prevent
evaporation of Iodine to visibly more invisible dots. Because the beaker was not wrapped and
cleaned well, the results were not as expected. The TLC plate did not show more dots.
Figure 4: TLC plate after stimulated by iodine.
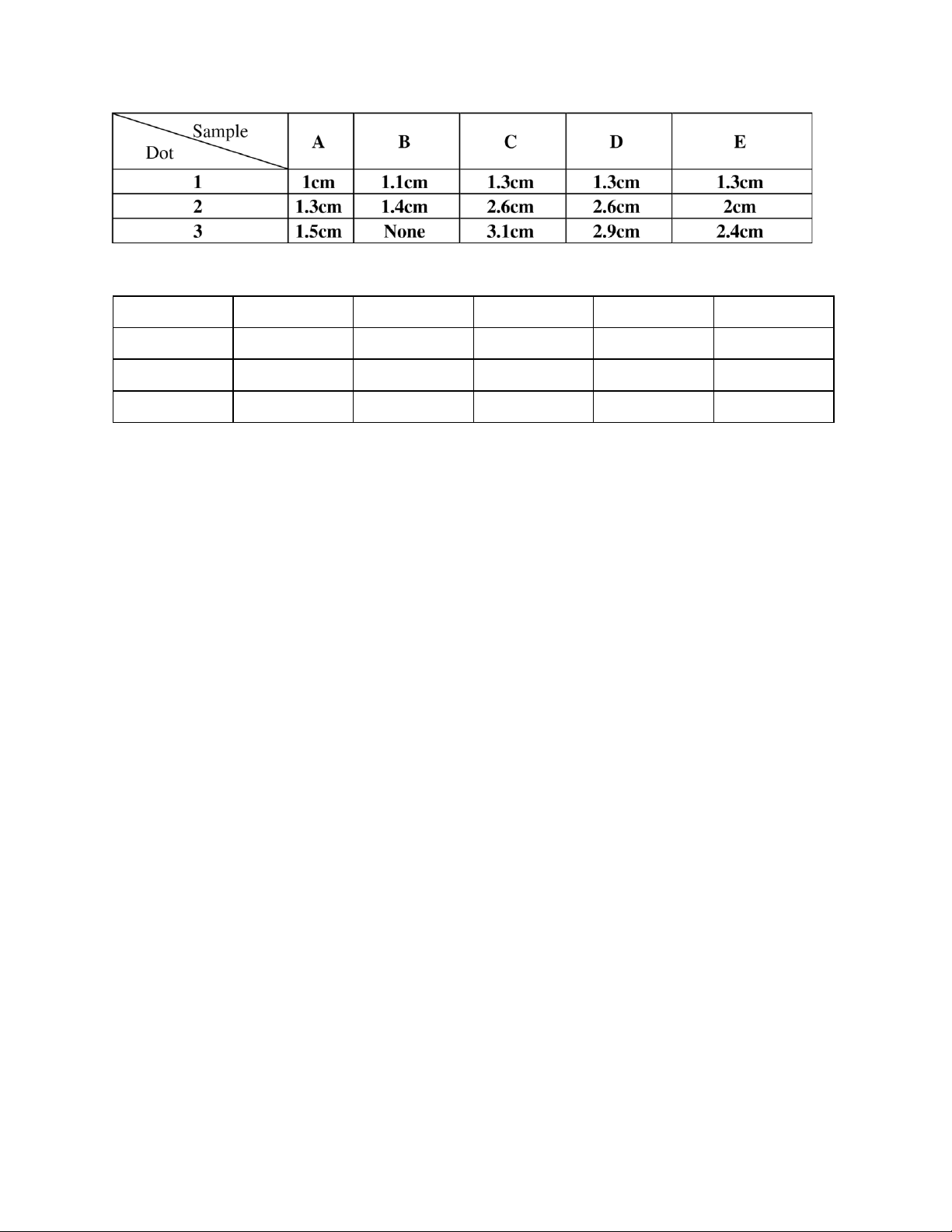
Table 1: Distance traveled by substances data. 6
Distance traveled by solvent front = 3.8cm
Table 2: Rf of substances data. A B C D E Rf1 0.26 0.29 0.34 0.34 0.34 Rf2 0.34 0.37 0.68 0.68 0.53 Rf3 0.39 None 0.82 0.76 0.63
As the distance traveled by a chemical increase, the corresponding Retention Factor (Rf) value also
increases, indicating a decrease in polarity. • Discussion:
In Thin-Layer Chromatography (TLC) with spinach leaf extracts, iodine is commonly used
as a visualization agent. This method allows for identification and quantification of compounds in
the extract that might not be detectable using UV-Visible spectroscopy. However, iodine can
evaporate easily and must be covered by aluminum foil.
Using two different solvents for extraction at the beginning of the lab serves the purpose of
separating and extracting several types of compounds from the sample of spinach leaf. Water, as
an aqueous solvent, is used to extract polar compounds, while organic solutions like methanol are
used to extract nonpolar compounds. By using both solvents, a more comprehensive information
of compounds present in spinach leaf can be obtained.
The purpose of using different methods of detection is to make it possible to figure out the
components in many conditions. UV visible are employed to identify and quantify different
components present. Apart from pigments like chlorophyll, spinach leaf extracts may contain
others such as amino acids, sugars, lipids and minerals like potassium and magnesium, which also can be detected. 7 V. CONCLUSION
To conclude, Thin Layer Chromatography was a laboratory technique that could identify
components that were found in a mixture. TLC also included a mobile phase which was a pure
solvent or a mixture of solvents. The configuration of the mobile phase depended on the polarities
of the compounds that were being separated. On the TLC plate, there was a movement of different
spots. There was capillary action of the mobile phase where it could elute up the TLC plate. The
portion of the plate near the solvent plate were less components where the polar components were
near the line of origin. The components that were less polar and more attracted to the solvent would
elute up the TLC plate meaning that these were not as attracted to the silica gel. VI. REFERENCES
• "High performance thin-layer chromatography (1977) HPTLC" by U.B. Hezel
• “Thin layer chromatography in spinach analysis: Journal of Liquid Chromatography &
Related Technologies (2018) “by V. Danciu
• “Thin-layer chromatography” by M. Santiago & Methods Enzymol (2013) 8



