





Preview text:
Table of Contents
I. ABSTRACT ............................................................................................................................... 3
II. INTRODUCTION ................................................................................................................... 3
III. MATERIALS AND METHODS ........................................................................................... 4
Materials .............................................................................................................................. 4
Methods ................................................................................................................................ 4
IV. RESULTS AND DISCUSSIONS ........................................................................................... 5
Result: ........................................................................................................................................ 5
Discussions ................................................................................................................................ 6
V. CONCLUSION .......................................................................................................................... 7
VI. REFERENCES ....................................................................................................................... 7
VII. LAB NOTES .......................................................................................................................... 7 2 I. ABSTRACT
Column chromatography was a versatile method employed for the separation and purification
of biomolecules, particularly proteins, possessing diverse properties such as size, shape, net charge,
and binding affinity. This technique involved the selective adsorption of a mixture of proteins onto
a stationary phase within a packed column. Upon the introduction of a mobile phase, the adsorbed
molecules migrate based on their affinity towards the solvent. The eluent, a mixture of analytes,
was pressurized and used as the mobile phase, facilitated by either liquid or gas pumping. The
stationary phase was created within the column by coating tiny particles or applying a thin film to
the inner walls. Analytes were subsequently obtained as eluates upon exiting the column, driven
by their distribution coefficients with the mobile phase. Those with higher affinity for the stationary
phase exhibit slower movement out of the column.
To ensure uniform packing of the beads, cotton wool was introduced at the column's base along
with the stationary phase. The beads were then added either as a slurry or in a dry state. The mobile
phase complements the stationary phase and entered the chromatographic system through the
injector. Elution was achieved using solvents selected based on their polarity, employing either
isocratic or gradient elution techniques. In the latter, a range of solvents with varying polarities
was used. Eluates were detected through the measurement of parameters utilizing visible UV. This
method found wide application, including the extraction of animal-derived insecticides, such as
waxes, pigments, and lipids. In the medical field, it played a crucial role in the synthesis of the
peptide hormone pramlintide for diabetes treatment. Additionally, column chromatography was
routinely utilized for separating impurities, purifying biological mixtures, isolating active
molecules, and extracting metabolites from various samples. II. INTRODUCTION
Column chromatography was a method by which the substances to be separated are put into
the upper part of a column that was filled with an adsorbent material. This technique involved the
differential adsorption of chemicals to an adsorbent material as they travel a column at different
velocities, which allows the separation into different fractions. Column chromatography was a
practical purification technique that can be employed both on a small and large scale for the 3
purpose of isolating and purifying materials intended for subsequent experimental usage. The
fundamental principle of column chromatography was the absorption of solutes from a solution
using a stationary phase, followed by the subsequent separation of the mixture into different components. III. MATERIALS AND METHODS Materials • 3 Vials • Cotton • Capillary • Silica plate for TLC • 1 Cylinder 5 mL • Ethanol • 1 Spatula • Distilled water • 1 Forceps • Aluminum foil • 3 Glass pipet • Hexane • 1 plastic pipet • Aceton • 1 Beaker 100 mL
• Silica for column chromatography Methods
This method of this report has 3 separate parts: prepare spinach leaf sample for column
chromatography, prepare the column as well as run the column, and identify the fractions. First, in
the spinach sample preparation part, collect the spinach supernatant from the spinach juice and
then, mix the supernatant with silica gel carefully and thoroughly (the silica gel weight should be
appropriate to make the mixture dry, coarse, and light color). Move to the Column preparation part,
use a manual way to get a small piece of cotton to the bottom of the column (the cotton piece
should be appropriate to prevent the sand and silica will not be dropped out from the bottom but
still able for the eluent to drip at an acceptable rate). After that, get the column to the ring stand
and rinse into the column with initial ethanol (always maintain the solution 1 cm from the silica
layer in the column). Then prepare a solution that combines dry silica and eluent with a ratio is
approximately 1:2, respectively, pour the prepared slurry into the column (put an effort into
maximizing the amount of silica that goes into the column by stirring the slurry). Moving to the
next step, let the solvent drip into a clean vial (try to tab on the side of the column to help the silica
settle uniformly). Finally, use a Pasteur pipet to rinse any silica remaining on the sides of the
column, the purpose is to let the silica settle. For the final part (run the column and identify the
fractions), collect the sample with 3 different colors from the column and perform it with TLC (use
the same technique as the previous experiment) against pure samples. The solvent for TLC methods
is a mixture of hexane and acetone (ratio 70:30 respectively). 4 IV.
RESULTS AND DISCUSSIONS • Result:
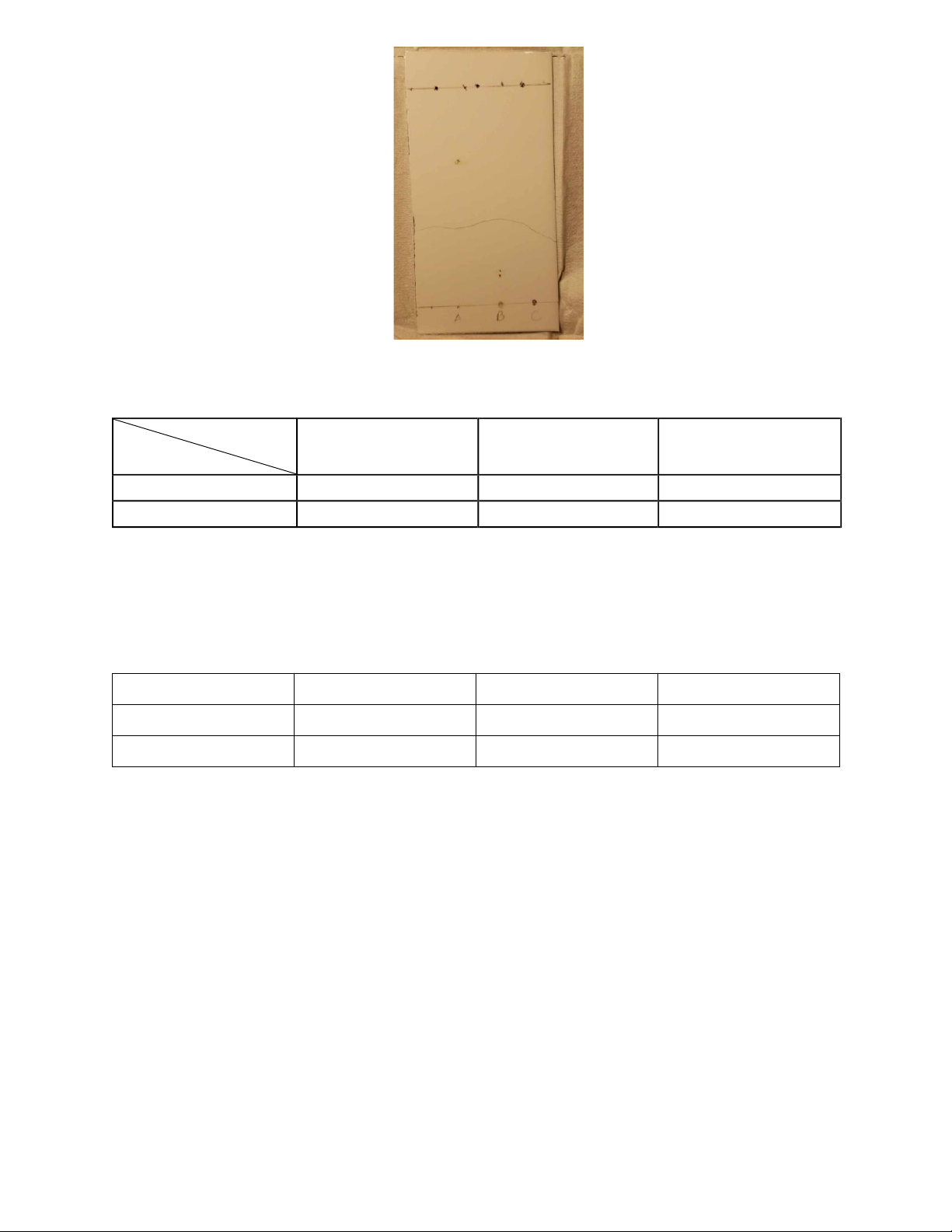
Table 1: Collected samples. Sample A Sample B Sample C
Following the preparation of a solvent mixture of 70% hexane and 30% acetone, the
thinlayer chromatography (TLC) plate was heated until the solvent had evaporated completely. It
was evident that sample A included one chemical substance, sample B had two, sample C did not appear any point.
Figure 1: TLC plate observation after dipping in 70:30 hexane-acetone.
Then, the TLC plate was stimulated by UV light to two dots at sample B and C. The new
features of sample B and sample C remained at the initial point. 5
Figure 2: TLC plate after stimulated by UV light.
Table 2: Distance traveled by substances data. Sample A B C Dot 1 5.2 cm 1 cm 0 cm 2 None 1.2 cm None
Distance traveled by solvent front = 2.7 cm
Table 3: Rf of substances data. A B C Rf1 1.926 0.37 0 Rf2 None 0.44 None • Discussions
In column chromatography, ethanol is a viable choice as a solvent for the separation of
pigments in spinach because its polarity enables the molecule to engage in interactions with other
polar molecules, such as pigment molecules polar components. It can dissolve polar compounds
and leave nonpolar compounds behind. It also allows the polar components to migrate and travel
up the TLC at a reasonable rate and promotes good separation. And it is not harmful to the
researcher when doing column chromatography.
After collecting three samples from the column and spotting a small amount of them in 6
TLC, then dipping TLC in 70:30 hexane-acetone and must be covered well with aluminum foil.
Because the beaker was not wrapped well, the solvent front was unstable as a straight line as
expected. The distance traveled by solvent front was the furthest point that solvent front traveled.
Put TLC under UV light to visualize the dots of three samples. Sample A and C had one
dot, as expected. Sample B had two dots which means the separation failed because of technical
issues. When collecting sample B, the column was not filled with more ethanol to maintain it 1cm
higher than the sample, so the separation failed. Always keep in mind that do not let the solvent
dry, the solvent must always be 1cm higher than the sample. V. CONCLUSION
To sum up, column chromatography was a good technique for separating organic
compounds in the Organic Chemistry field. When separating compounds in the column, the sample
must be always filled with solvent, which was 1cm higher than the sample. If the solvent dried, the
separation failed. Sample A and C were successfully separated, which presented one dot on TLC.
Meanwhile, sample B failed due to the solvent running dry. When dipping the TLC in 70:30
hexane-acetone, it must be wrapped tightly to stabilize the solvent front. If it was not wrapped well,
the solvent front would fluctuate. VI. REFERENCES
Advances in extraction technologies: isolation and purification of bioactive compounds from
biological materials. (2020, October 9). Advances in Extraction Technologies: Isolation and
Purification of Bioactive Compounds From Biological Materials - ScienceDirect.
Chromatographic techniques: types, principles, and applications. (2021, October 22).
Chromatographic Techniques: Types, Principles, and Applications - ScienceDirect. VII. LAB NOTES 7



