






Preview text:
Table of Contents I.
ABSTRACT ........................................................................................................................................ 3 II.
INTRODUCTION .............................................................................................................................. 3
III. MATERIALS AND METHODS ....................................................................................................... 3 •
Materials: .......................................................................................................................................... 4 •
Method: ............................................................................................................................................. 4
IV. RESULT AND DISCUSSION .......................................................................................................... 4 •
Result: ............................................................................................................................................... 5 •
Discussion: ........................................................................................................................................ 6 V.
CONCLUSION .................................................................................................................................. 7
VI. REFERENCES................................................................................................................................... 7
VII. LAB NOTES ....................................................................................................................................... 7 I. ABSTRACT
Reflux is a process that entails heating a mixture or solution inside a closed container, such
as a flask, and then condensing the vapors. After that, the condensed liquid is then returned to the
system to either heat up again or react with other chemicals. This experiment can be used to keep
the temperature and product or reactant concentrations constant, particularly when the reaction is
slower or demands elevated temperatures. When heating solution in an open vessel will result in 2
solvent loss; however, a reflux apparatus prevents this. The reactant concentration is maintained at
all times in a reflux setup because the condenser remains solvent vapors. Reflux can also help in
clearing the reaction mixture of undesirable products or contaminants. The flask contains the
reaction mixture, the condenser cools down the vapors and returns them to the flask, and the heat
maintains the boiling point of the solvent or solution.
Refluxing a solution is mostly used to keep it heated at a steady temperature for extend
among of time without losing any solvent or reactant. This makes it possible for the reaction to
finish and the formation of the desired product. This technique is used in many diverse types of
organic reactions such as electrophilic aromatic substitution, nitration, and oxidation. II. INTRODUCTION
Reflux is a technique used to isolate compounds from intricate mixtures over an extended
period of time. It includes the process of vapor condensing and returning the condensate to the
original system. Utilizing specialized glassware, including a reflux condenser, we create a closed
system that optimizes the extraction process. The main purpose of this technique is to heat a
solution in a controlled manner at a constant temperature. Acetic acid, petroleum ether, diethyl
ether, and flaked coconut are the substances used in this experiment. The investigation will explore
the theoretical foundations of the technique, highlighting the significance of solvent choice and
accurate regulation of reflux settings.
Reflux conditions are commonly used when using petroleum ether, a mixture of lowboiling
hydrocarbons obtained from petroleum ether, as a solvent for non-polar processes. It is suitable for
some reactions that are needed for higher temperatures but not also high that the solvent is overly
evaporated because of its low boiling point. III. MATERIALS AND METHODS • Materials:
Reflux apparatus with stand, heating mantle Diethyl ether and water pump Acetic acid 1 Round-bottom flask 250 mL 1 Beaker 100 mL TLC Forceps Capillary Lamp 3 Flaked coconut. Aluminum foil Petroleum ether
Thin Layer Chromatography (TLC) • Method:
The methods of this experiment will be divided into two parts: Maceration of petroleum
ethers and TLC of obtained products. In the first part, transfer 15 g of flaked coconut into a 250
mL round-bottom flask, top it over with 100 mL of petroleum ether, and let it reflux for one hour.
After that, allow the mixture to cool, then use a funnel to strain the coconut shreds from the solution
using filter paper. Then, weigh using a rotary evaporator after concentrating the resulting solution
under lower pressure. In the TLC of the obtained products part, on a silica plate, develop the
resultant product in a chamber that has been saturated with petroleum ether: diethyl ether, and
glacial acetic acid (80:20:10). After removing the plate, let the solvent drain. Then, after drying
use a hot plate and gently heat the TLC plate. The amount and physical characteristics of the
collected coconut are reported in a summary. IV. RESULT AND DISCUSSION 4 • Result:
After reflux extraction, we spot 2 points A and B in the Thin-layer chromatography (TLC).
Point A is coconut oil and point B is the sample obtained.
Figure 1: TLC plate after spotting coconut oil and sample.
When dipping in develop solvent, only the solvent increased. Following a drop in the
developed solution, no new point emerged.
Figure 2: TLC plate after soaking in develop solution.
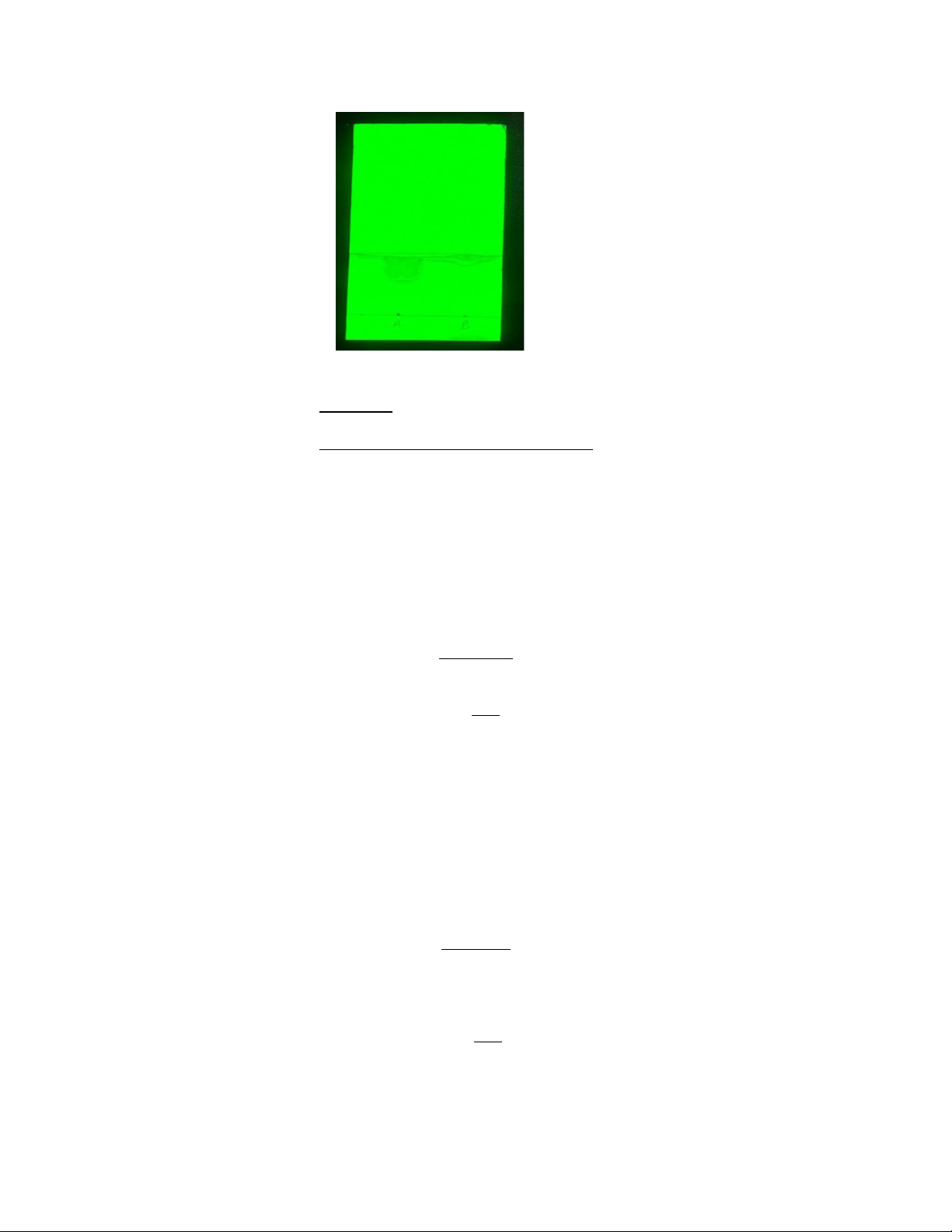
In figure 3 illustrates the variations between the sample and coconut oil under UV-light. 5
Figure 3: TLC plate under UV-visualization 𝑅𝑓 =
𝑑𝑖𝑠 tan 𝑐 𝑒 𝑡𝑟𝑎𝑣𝑒𝑙𝑒𝑑 𝑏𝑦 𝑠𝑜𝑙𝑣𝑒𝑛𝑡 𝑓𝑟𝑜𝑛𝑡𝑑𝑖𝑠 tan 𝑐
𝑒 𝑡𝑟𝑎𝑣𝑒𝑙𝑒𝑑 𝑏𝑦 𝑠𝑢𝑏 tan 𝑒
Distance traveled by solvent front = 2.6cm
Distance traveled by substance of sample A = 1.5cm 𝑅𝑓𝐴 = 1.5cm = = 0.58 2.6 𝑐𝑚
Distance travelled by substance of sample B = 2.1cm • Discussion:
𝑅𝑓𝐵 = 2.1cm___ = 0.81 2.6 𝑐𝑚 6
The solvent system selection was one of the most important factors in this investigation.
Based on their individual qualities, a certain ratio of petroleum ether, diethyl ether, and acetic acid
was chosen. The non-polar components in the flaked coconut were to be dissolved using petroleum
ether, a non-polar solvent. While acetic acid offered an acidic medium that could extract specific
classes of chemicals selectively, diethyl ether, with its intermediate polarity, helped with the
extraction of a wider variety of compounds.
When performing reflux extraction, it is important to notice the heating temperature.
Maintain the constant temperature after a few first bubbles appear. Do not set the temperature too
high because too much solvent evaporate can cause the loss of the liquid components.
The distance traveled by the substance of sample A is determined as its lowest. The 𝑅𝑓𝐴 is
smaller than 𝑅𝑓𝐵 (0.58<0.81), so the substance of sample B is less polar than the substance of 𝑅𝑓𝐴 < 𝑅𝑓𝐵
sample A. Both samples A and B are coconut oils but . Due to sample A was developed so much
that its dot was spread out because of the diffusion. V. CONCLUSION
In this reflux extraction experiment aimed at isolating fatty acids and lipids from flaked
coconut, the use of petroleum ether as a solvent in the reflux system proved to be an effective and
efficient method. The continuous reflux process, marked by the vaporization and condensation of
petroleum ether, facilitated prolonged contact between the solvent and the sample, enhancing the
extraction of lipids components. 7
Comparative analysis of the obtained outcomes against the anticipated theoretical values,
derived from the established composition of flaked coconut, facilitated an assessment of the
extraction's success. Regrettably, unforeseen procedural oversights hindered the observation of the
final product on the TLC plate. This investigation not only substantiated the efficacy of reflux
extraction in segregating lipids and fatty acids but also established a foundational understanding of
the critical role of polarity alignment in chemical experiments.
In conclusion, the reflux reaction experiment has made significant contributions to our
knowledge of organic components and polarity match by shedding light on the extraction of fatty acids and lipids. VI. REFERENCES
Özenoğlu, A., Anul, N., & Özçelikçi, B. (2023, September 1). The relationship of gastroesophageal
reflux with nutritional habits and mental disorders. Human Nutrition & Metabolism. 8 VII. LAB NOTES
Figure 4: Lab notes 6th December 9




