




Preview text:
Contents
I. ABSTRACT ............................................................................................................................... 3
II. INTRODUCTION ................................................................................................................... 3
III. MATERIAS AND METHODS ............................................................................................. 4
• Materials: ..........................................................................................................................4
• Methods: ...........................................................................................................................4
IV. RESULT AND DISCUSSION ................................................................................................ 5
• Result: ...............................................................................................................................5
• Discussion: .........................................................................................................................6
V. CONCLUSION ......................................................................................................................... 6
VI. REFERENCES ....................................................................................................................... 7
VII. LAB NOTES ......................................................................................................................... 7 2 I. ABSTRACT
The method of simple distillation involved heating and cooling liquids to separate and
purify them. It was used to separate liquids from solids or nonvolatile components or when the
boiling temperatures of two liquids differed noticeably from one another. The most volatile
component of a mixture was heated in a simple distillation process to turn it from a liquid into
vapor. In this experiment, a mixture of cyclohexane and toluene was distilled using a simple
distillation apparatus. The composition and temperature of the vapor and liquid phases were
monitored and plotted on a boiling point-composition diagram. The results showed that the
distillate was enriched in cyclohexane, the lower boiling component, while the residue was
enriched in toluene, the higher boiling component. The experiment demonstrated the principles
and applications of simple distillation. Like reflux, however with this technique, the condensing
liquid was collected in a different container rather than falling back into the pot. When the
components of a liquid combination had different boiling points, this approached could be used to
separate them. Distillation came in several forms for a variety of uses. Liquids with at least 100
degrees in their boiling points were separated by simple distillation.
In this method, the test tube contained liquids that had a much higher boiling point. Simple
distillation was useful for separating liquids that had an enormous difference in boiling points, such
as water and salt, or ethanol and water. However, it was not effective for separating liquids that
had similar boiling points, such as ethanol and acetone, or benzene or toluene. For these mixtures,
had to use other methods, such as fractional or steam distillation. II. INTRODUCTION
Distillation is a technique used by chemists to separate components of a liquid mixture with
different boiling points. To do so, the liquid mixture is heated to only boil one component, which
separate from the mixture as a gas. This gas then passes through a cold tube, condensing it back
into liquid and flowing into a separate vessel. Simple distillation involve performing this procedure
once to separate two liquids with different boiling points. In simple distillation, a mixture is heated
to change the most volatile component from a liquid into vapor. The vapor increase into a condenser.
Simple distillation could be used when the boiling points of two liquids were significantly different
from each other or to separate liquids from solids or nonvolatile components. 3 III. MATERIAS AND METHODS • Materials:
− Distillation apparatus with thermometer, stand, heating mantle and water pump
− 1 Round-bottom flask 100 mL − 1 Water bath − 2 Glass pipet − 2 Cylinder 10 mL − 10 Test tubes and rack − Aluminum foil − Refractometer − Hexane − Toluene − Distilled water • Methods:
Firstly, fill a 100 mL round-bottom flask with 25 mL each of hexane and toluene. Then,
clamp the flask to a ring stand and support it with a water bath. Put eleven identically sized, dry,
and clean test tubes together: 5mL of water to one place it within a rack for test tubes. This test
tube's liquid capacity is used to calculate the volumes of the distillation fractions (by comparison).
After that, plug a rheostat into the wall socket after plugging the heating mantle into it. This mixture
should boil at a setting of four. Raise or lower the voltage setting to produce a continuous boil that
keeps the distillate's drop rate at around one drops every 2 seconds (The very first 1 mL portion is
important! Collect it carefully and run its refractive index right away). When the temperature starts
to drop or fluctuate and the distillation flask is nearly empty, the process is finished. (The final
portion will not be 5 mL due to a hold-up.) Once the device has cooled, pour the residue into a
small, graduated cylinder, and note the volume. Along with the refractive indices of the initial
hexane and toluene, measure the refractive index of each fraction. 4 IV. RESULT AND DISCUSSION • Result:
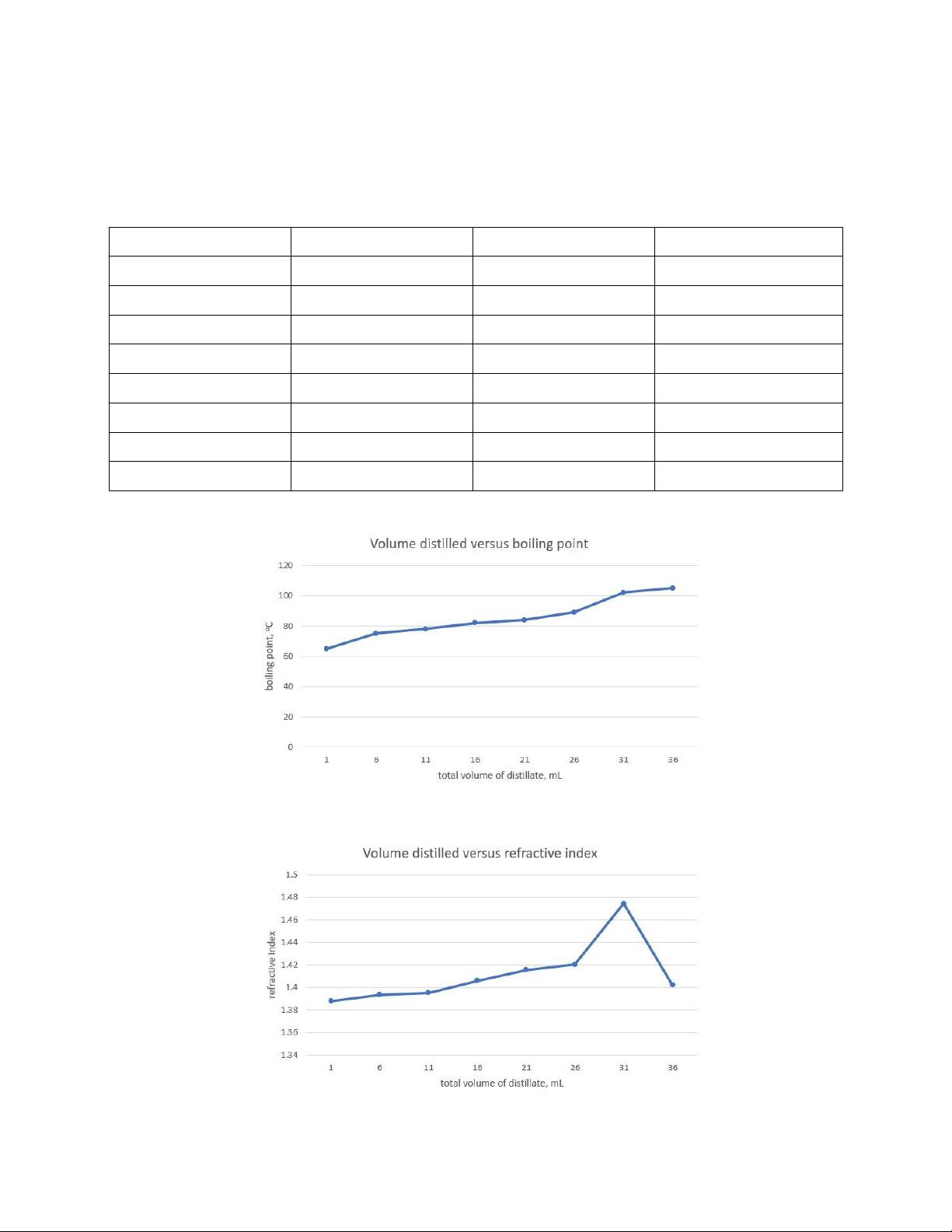
Table 1: Data record of experiment. Test tube Volume of distillate Temperature Refractive index 1 1mL 65oC 1.3880 2 5mL 75oC 1.3936 3 5mL 78oC 1.3953 4 5mL 82oC 1.4059 5 5mL 84oC 1.4156 6 5mL 89oC 1.4203 7 5mL 102oC 1.4745 8 5mL 105oC 1.4022
Figure 1: Phase diagram of boiling point versus mL distilled. 5
Figure 2: Phase diagram of refractive index versus mL distilled. • Discussion:
In this experiment, the efficacy of a two-step distillation procedure for separating products
with high boiling points during chemical reactions was evaluated. By removing the solvent from
the reaction mixture during the first distillation, contamination was decreased, and a more
concentrated residue was produced. The residue was then subjected to a second distillation in a
smaller flask with the aim of achieving the necessary level of purification. This method of
successive distillation sped up the removal of a significant solvent volume and provided exact
control over the separation process. The effectiveness and applicability of the approach were
emphasized, highlighting its potential to optimize product separation in industrial and laboratory settings.
Some pumice must be added in the round-bottomed flask with the mixture to create boiling
sprouts. It must have boiling sprouts for the solution to boil. If there is no boiling point, the solution
will not boil even though the temperature has exceeded its boiling point. At some point, the
roundbottomed flask will break because the solution suddenly boils at the same time.
After finishing the experiment, carefully remove the equipment and put the remaining
chemicals into the organic chemicals waste tank. It takes up to 20 minutes for the equipment to
cool down and readily be cleaned up with water. If the equipment has not been cooled down enough,
it will be broken due to the sudden change in temperature when it is cleaned with water.
In this case, the mercury thermometer broke while being cleaned. V. CONCLUSION
In conclusion, the simple distillation experiment substantiates its efficacy in the
segregation of components characterized by distinct boiling points. The method, having facilitated
the extraction of a target product from a complex mixture, attests to its applicability in diverse
chemical contexts. The discourse underscores nuanced considerations including challenges
pertaining to purity, the advantageous prospect of solvent recovery, and the critical role of
temperature control. The potential influence of personal errors on precision is acknowledged,
emphasizing the indispensability of meticulous procedural adherence. Overall, the experiment
contributes valuable insights into the fundamental principles and practical applications of simple 6
distillation, warranting further scholarly exploration for potential refinements and tailored
adaptations within industrial and laboratory frameworks. VI. REFERENCES
Analytical expression and its application of Rayleigh fractionation for ternary mixtures
2023, Chemical Engineering Science.
Estimating mixture properties from batch distillation using semi-rigorous and rigorous
models 2019, Computer Aided Chemical Engineering. VII. LAB NOTES
Figure 3: Lab notes on November 8th, 2023 7



