

















Preview text:
lOMoAR cPSD| 58562220
VIETNAM NATIONAL UNIVERSITY - HCMC INTERNATIONAL UNIVERSITY
School of Biotechnology – Department of Food Technology Laboratory Manual PRACTICE IN FOOD
MICROBIOLOGY BTFT254IU
Instructor: MSc. Nguyen Thi Huong Giang
Teaching Assistant: BEng. Nguyen Thanh Nhi
Ho Chi Minh City, 2024 Contents
LAB PROGRESS ........................................................................................................................... 4
EVALUATION METHOD ............................................................................................................ 5
LABORATORY 1 .......................................................................................................................... 6
MICROBIOLOGICAL CULTURE MEDIA PREPARATION AND STERILIZATION ............ 6 lOMoAR cPSD| 58562220
1. Objective .............................................................................................................................. 6
2. Material ................................................................................................................................ 6 2.1.
Equipment ................................................................................................................... 6 2.2.
Media .......................................................................................................................... 6
3. Background .......................................................................................................................... 7 3.1.
Culture Media Composition ........................................................................................ 7 3.2.
Media classification .................................................................................................... 7 3.3.
Preparation of culture media ....................................................................................... 9 3.4.
Pouring a plate ............................................................................................................ 9 3.5.
Storage of media ....................................................................................................... 10 3.6.
Sterilization of Media ............................................................................................... 10
4. Procedure ............................................................................................................................ 10 4.1.
Prepare solid media ................................................................................................... 10 4.2.
Prepare liquid medium .............................................................................................. 11 4.3.
Prepare diluent .......................................................................................................... 13 4.4.
Procedure for autoclaving ......................................................................................... 14
5. References .......................................................................................................................... 14 LABORATORY 2
........................................................................................................................ 15
SAMPLING, SAMPLE PREPARATION, AND CULTURE technique ..................................... 15
1. Objective ............................................................................................................................ 15
2. Material .............................................................................................................................. 15 2.1. Equipment
................................................................................................................. 15 2.2.
Media ........................................................................................................................ 15 2.3.
Sample....................................................................................................................... 15
3. Background ........................................................................................................................ 16 lOMoAR cPSD| 58562220 3.1.
Samples must be representative for the entire lot ..................................................... 16 3.2.
Collecting samples for analysis ................................................................................ 17 3.3.
Dilution series ........................................................................................................... 18 3.4.
Culture technique ...................................................................................................... 20
4. Procedure ............................................................................................................................ 21 4.1.
Using aseptic technique to do a sampling of milk product and make a dilution series
(Total aerobic count). ............................................................................................................. 21 4.2.
Using aseptic technique to do a sampling of grapes and make a dilution series
(Yeast/mold determination).................................................................................................... 25 4.3.
Using aseptic technique to do a sampling of juice and make a dilution series
(Enumeration of Coliform)..................................................................................................... 27
5. References .......................................................................................................................... 27 LABORATORY 3
........................................................................................................................ 29
SUBCULTURE METHOD AND STANDARD PLATE COUNT ............................................. 29
1. Objective ............................................................................................................................ 29
2. Material .............................................................................................................................. 29
3. Principle .............................................................................................................................. 29 3.1.
Subculture method .................................................................................................... 29 3.2.
Slant culture and stab culture .................................................................................... 30 3.3.
The plate count method ............................................................................................. 30
4. Procedure ............................................................................................................................ 33 4.1.
Prepare TSI agar slant ............................................................................................... 33 4.2.
PCA counting ............................................................................................................ 34 4.3.
Sub-culturing incubated LST into tube containing BGBB (Enumeration of Coliform) 34 4.4.
Streaking the agar slant surface and stabbing the butt (Salmonella confirmation) ... 35
5. References .......................................................................................................................... 36 LABORATORY 4
........................................................................................................................ 36 lOMoAR cPSD| 58562220
BIOCHEMICAL ACTIVITY OF BACTERIA AND MOST PROBABLE NUMBER
TECHNIQUE ................................................................................................................................ 36
1. Objective ............................................................................................................................ 36
2. Material .............................................................................................................................. 36
3. Principle .............................................................................................................................. 36 3.1.
Biochemical activity of bacteria – Carbohydrate fermentation - Triple Sugar Iron
Agar Test ................................................................................................................................ 36 3.2.
Most probable number technique (MPN) ................................................................. 37
4. Procedure ............................................................................................................................ 41 4.1.
Interpret the TSI test for Salmonella confirmation. .................................................. 41 4.2.
Verification of Coliform’s presence and calculation of the most probable number . 41
5. References .......................................................................................................................... 41 LABORATORY 5
........................................................................................................................ 42
BACTERIAL MORPHOLOGY AND STAINING ..................................................................... 42
1. Objective ............................................................................................................................ 42
2. Material .............................................................................................................................. 42
3. Background ........................................................................................................................ 43 3.1.
Bacterial smear.......................................................................................................... 43 3.2.
Staining ..................................................................................................................... 43
4. Procedure ............................................................................................................................ 44 4.1.
Smear Preparation ..................................................................................................... 44 4.2.
Simple staining.......................................................................................................... 45 4.3.
Gram stain ................................................................................................................. 45
5. References .......................................................................................................................... 47 APPENDIX 1
................................................................................................................................ 48 lOMoAR cPSD| 58562220
MICROORGANISM DETERMINATION FLOWCHART ........................................................ 48
APPENDIX 2 ................................................................................................................................
52 EVALUATION METHOD .......................................................................................................... 52 LAB PROGRESS Week Task 1
- Preparation and sterilization of BPW, PCA, LST, BGBB
- Sampling of milk sample with pouring and spreading technique for PCA test
- Sampling of juice sample, culturing to LST, and subculturing to BGBB 2
- Preparation and sterilization of PW, TSI, PCA, DRBC - Counting PCA - Streaking PCA
- Estimation of total coliforms with most probable number (MPN) test
- Sampling of grape samples and culturing to DRBC - Stabbing TSI 3 - TSI analysis - Counting DRBC - Gram staining (PCA, BGBB)
- Motility examination of Salmonella 4 - Oral examination EVALUATION METHOD 1. Oral exam (40%)
The oral exam has 3 types of questions for each student: lOMoAR cPSD| 58562220 - Theory questions - 25%
- Result analysis questions - 35%
- Practice questions (You will be asked to do any labwork skill that you have learned during the course) - 30% 2.
Report (50%): (5 types of microorganisms: Total aerobic count, coliforms, Salmonella,
Yeast & Mold, Morphology of LAB & Salmonella)
Each microorganism will follow the below report structure - Introduction - Material and Methodology - Result & Discussion - Conclusion - References 3. Lab behavior (10%) LABORATORY 1
MICROBIOLOGICAL CULTURE MEDIA PREPARATION AND STERILIZATION 1. Objective
Each student should be able to
− Describe the different types of culture media and their composition, and give several examples
of what each is used for
− Describe the various ways culture tubes are capped
− Describe how to prepare and transfer culture media
− Prepare defined and undefined media, and prepare agar plates
− Describe the concept of sterility
− Describe how various media, supplies, and equipment can be sterilized −
Correctly and safely use the autoclave 2. Material lOMoAR cPSD| 58562220 2.1. Equipment
Test-tube rack, Screw capped test tube; 2-liter Erlenmeyer flask; Petri dish; Pasteur pipette; Stirring
bar; Micropipette; Beaker 500 ml, 1000 ml; Cylinder 100 ml; Alcohol Burner; Thermostatic water
bath, balance, incubator, autoclave, heating oven, biological safety cabinet, pH meter, vortex
mixer, heat-proof Zetex fabric gloves, weighing paper, aluminum foil, chemical storage hood 2.2. Media
− Buffered peptone water (BPW)
− Brilliant Green Bile Lactose Broth 2% (BGBB)
− Chloramphenicol Selective Supplement
− Dichloran Rose Bengal Chloramphenicol Agar (DRBC Agar)
− Lauryl Sulphate Tryptose Broth (LST) lOMoAR cPSD| 58562220 − Peptone water (PW) − Plate count agar (PCA)
− Rappaport Vassiliadis Soya Broth (RVS) (for TA)
− Xylose Lysine Desoxycholate Agar (XLD) (for TA) 3. Background
3.1. Culture Media Composition
− A microbiological culture medium is a substance that encourages the growth, support, and survival of microorganisms. − Main components: + Water
+ N-source: proteins, peptides, amino acids
+ Energy source: carbohydrates, proteins
+ Additional growth factors: vitamins, minerals, trace elements, an organic salt
− Culture media can be used in different manners: + In test-tubes + In flasks + In petri dishes
3.2. Media classification
3.2.1. Chemical constituents (defined or complex)
Figure 1.1a. A chemically defined medium lOMoAR cPSD| 58562220
Figure 1.1b. A complex (undefined) medium
3.2.2. Physical nature (liquid, semi-solid or solid) 1.2a. Liquid medium 1.2b. Solid medium 1.2c. Semi-solid medium (Source: Wikimedia) (Source: Differencebetween) (Source: Differencebetween)
Figure 1.2. Different types of culture media
− Agar medium (solid): are used for isolating bacteria or for determining the colony characteristics of the microorganisms.
− Liquid medium: are used for various purposes such as propagation of a large number of organisms,
fermentation studies, and various other tests. 3.2.3. Function
− Basal media (non-selective media): may be used for growth (culture) of bacteria that do not
need enrichment of the media. Example: Nutrient broth, nutrient agar and peptone water.
− Enriched media: Addition of extra nutrients in the form of blood, serum, egg yolk, etc., to
basal medium makes enriched media. Enriched media are used to grow nutritionally bacteria.
Example: Blood agar, chocolate agar.
− Selective and enrichment media: Selective media allow certain types of organisms to grow lOMoAR cPSD| 58562220 −
and inhibit the growth of other organisms. While selective media are agar based, enrichment media
are liquid in consistency. Both these media serve the same purpose.
Selectivity is accomplished in several ways. Organisms that can utilize a given sugar are easily
screened by making that sugar the only carbon source in the medium. On the other hand,
selective inhibition of some types of microorganisms can be achieved by adding dyes,
antibiotics, salts or specific inhibitors which affect the metabolism or enzyme systems of the
organisms. For example, media containing potassium tellurite, sodium azide or thallium
acetate (at concentrations of 0.1-0.5 g/l) will inhibit the growth of Gram-negative bacteria.
Media supplemented with penicillin (5-50 unit/ml) or crystal violet (2 mg/l) will inhibit the
growth of Gram-positive bacteria. Tellurite agar, therefore, is used to select for Gram-positive
organisms, and nutrient agar supplemented with penicillin can be used to select for Gramnegative organisms.
− Indicator (Differential) media are used to differentiate closely related organisms or groups of
organisms. Owing to the presence of certain dyes or chemicals in the media, the organisms will
produce characteristic changes or growth patterns that are used for identification or differentiation.
3.3. Preparation of culture media
Rehydrate powder according to manufacturer’s instructions. Before sterilization, ensure
ingredients are completely dissolved, using heat if necessary. Normally 15-20 cm3 medium per
petri dish. Dispense in volumes appropriate for sterilization in the autoclave/pressure cooker.
Agar slopes are prepared in test tubes or Universal/McCartney bottles by allowing sterile molten
cooled medium to solidify in a sloped position. 3.4. Pouring a plate
− Collect one bottle of sterile molten agar from the water bath or after cooling from autoclave
− Hold the bottle in the right hand; remove the cap with the little finger of the left hand
− Flame the neck of the bottle lOMoAR cPSD| 58562220
− Lift the lid of the Petri dish slightly with the left hand and pour the sterile molten agar into the
Petri dish and replace the lid
− Flame the neck of the bottle and replace the cap
− Gently rotate the dish to ensure that the medium covers the plate evenly lOMoAR cPSD| 58562220 − Allow the plate to solidify 3.5. Storage of media
Store stocks of prepared media at room temperature away from direct sunlight. Media in vessels
closed by cotton wool plugs/plastic caps that are stored for future use will be subject to evaporation
at room temperature; avoid wastage by using screw cap bottles.
Re-melt stored agar media in a boiling water bath, pressure cooker or microwave oven. Once
melted, agar can be kept molten in a water bath at 50°C until it is ready to be used. Sterile agar
plates can be pre-poured and stored in well-sealed plastic bags (media-containing base uppermost
to avoid heavy condensation on lid).
3.6. Sterilization of Media
There are 3 basic methods to sterilize media.
− Autoclaving: 15 min at 121oC
− Boiling: applied for heat sensitive media since 121oC can damage important components
− Bacteriological filter: physically removes bacteria and larger microorganisms from the solution
and thereby sterilizes them without heat (ø 0.22 µm) 4. Procedure
4.1. Prepare solid media
4.1.1. Plate Count Agar (PCA) (Total aerobic count)
− Prepare 3 parts of PCA (one for spread plating, one for pour plating and one for streaking) −
Suspend 23.5 g in 1000 ml of distilled water lOMoAR cPSD| 58562220
− Heat to boiling to dissolve the medium completely
Sterilize by autoclaving at 15 lb pressure (121oC) for 15 min
− Cool to 45-50oC and pour into sterile Petri plates for spread plating and streaking parts
− When the plates are cool (agar solidified), invert them to prevent condensing moisture from
accumulating on the agar surfaces
4.1.2. Dichloran Rose Bengal Chloramphenicol Agar (DRBC Agar) - Yeast/Mold determination
− Suspend 15.75 g in 500 ml of distilled water
− Heat to boiling to dissolve the medium completely
− Sterilize by autoclaving at 121oC for 15 min
− Cool to 50oC and aseptically add sterile reconstituted contents of 1 vial of chloramphenicol selective supplement
− Mix well and pour into sterile Petri plates
− When the plates are cool (agar solidified), invert them to prevent condensing moisture from
accumulating on the agar surfaces
4.2. Prepare liquid medium
4.2.1. Lauryl Tryptose Broth (LST) – selective enrichment medium - Coliform determination lOMoAR cPSD| 58562220 − − Composition Ingredients Weight (g) Tryptose 20 Lactose 5 Sodium chloride 5 Dipotassium hydrogen 2.75 phosphate Potassium dihydrogen 2.75 phosphate Sodium lauryl sulphate 0.1 Water 1000 ml
− Dissolve the different components in the water, by heating if necessary
− Adjust the pH, if necessary, so that after sterilization it is 6.8 ± 0.2 at 25°C
Dispense 10 ml of the broth into 9 tubes containing Durham − tubes
− Place the tubes in a test-tube rack or basket and place in the autoclave
− Sterilize by autoclaving at 121oC for 15 min (the Durham tubes shall not contain air bubbles after sterilization)
4.2.2. Brilliant Green Bile Broth (BGBB) - Coliform determination
− Suspend 40.01 g in 1000 ml of distilled water lOMoAR cPSD| 58562220
− Heat if necessary to dissolve the medium completely lOMoAR cPSD| 58562220
− Dispense the medium in quantities of 10 ml in test tubes containing Durham tubes
− Sterilize by autoclaving at 121oC for 15 min (the Durham tubes shall not contain air bubbles after sterilization) 4.3. Prepare diluent
4.3.1. Buffered Peptone Water (BPW) (total aerobic, coliform)
− Suspend 20 g in 1000 ml of distilled water (heat if necessary to dissolve the medium completely)
− Sterilize by autoclaving at 15 lb pressure (121oC) for 15 min
− Mix well and dispense into sterile flasks as desired
4.3.2. Peptone Water (PW) (Yeast/ mold)
− Suspend 1 g of dehydrated medium in 1000 ml of distilled water
− Mix well to dissolve the medium completely
− Sterilize by autoclaving at 121oC for 15 min
4.4. Procedure for autoclaving lOMoAR cPSD| 58562220
− TA will demonstrate the use of the autoclave.
− There will be 2 batches of autoclaving (1st batch: solid media, petri plates, some equipment;
2nd batch: BPW and PW in test tubes, other equipment).
− Load the autoclave with the freshly prepared culture media
− Close and lock the autoclave door
− Set the autoclave time for 15 min or longer and select a slow rate of exhaust
− Make certain that the autoclave temperature is set to 121oC
− Start the autoclave by pushing the start button or twisting the knob to the start position
− When the period of sterilization is completed and the pressure in the chamber reads 0, carefully
open the door and remove the containers, using heat-proof gloves 5. References
Burdass, D., Grainger, J., & Hurst, J. (2005). Basic practical microbiology: A manual. Reading,
U.K.: Society for General Microbiology.
L. Baert (2008) Food microbiology and analysis Practical work presentation. Gent University.
Culture of Microorganisms: 5 Steps. (n.d.). Retrieved May 14, 2020, from
http://www.biologydiscussion.com/microorganisms/culture-microorganisms/culture-
ofmicroorganisms-5-steps/31361
Harley, J., & Prescott, L. M. (2002). Laboratory Exercises in Microbiology (5th ed.). The McGraw−Hill. How Microbes Grow. (n.d.). Retrieved May 14, 2020, from
https://courses.lumenlearning.com/microbiology/chapter/how-microbes-grow/
Sandle, T. (2014, June 18). Assessment of Culture Media in Pharmaceutical Microbiology. Retrieved
from https://www.americanpharmaceuticalreview.com/Featured-
Articles/163589Assessment-of-Culture-Media-in-Pharmaceutical-Microbiology/ LABORATORY 2
SAMPLING, SAMPLE PREPARATION, AND CULTURE TECHNIQUE 1. Objective
Each student should be able to lOMoAR cPSD| 58562220
− Collect samples as representative for the entire lot − Do an aseptic sampling − Do dilution series 2. Material 2.1. Equipment
Blender, sterile plastic bag, Mortar and pestle; Scissor; Screw capped test tube; Erlen; Petri dish;
Pasteur pipette; alcohol jar; spreader; Micropipette; Beaker 500 ml, 1000 ml; Cylinder 100 ml;
Alcohol Burner; Thermostatic water bath, balance, incubator, autoclave, heating oven, biological
safety cabinet, pH meter, vortex mixer. 2.2. Media
− Sterilized Brilliant Green Bile Broth (BGBB)
− Sterilized Buffered Peptone Water (BPW)
− Sterilized Dichloran Rose Bengal Chloramphenicol Agar (DRBC Agar)
− Sterilized Lauryl Tryptose Broth (LST)
− Sterilized Peptone Water (PW)
− Sterilized Plate count agar (PCA) 2.3. Sample − Milk − Grapes − Juice 3. Background
3.1. Samples must be representative for the entire lot
− Sampling is to collect food samples that are representative and then to ensure that changes in
composition do not take place between collection and analysis.
− Food can be packed in different ways: + In batch
+ In separate units (bottles, cans, boxes)
Use a table with random numbers to collect. lOMoAR cPSD| 58562220
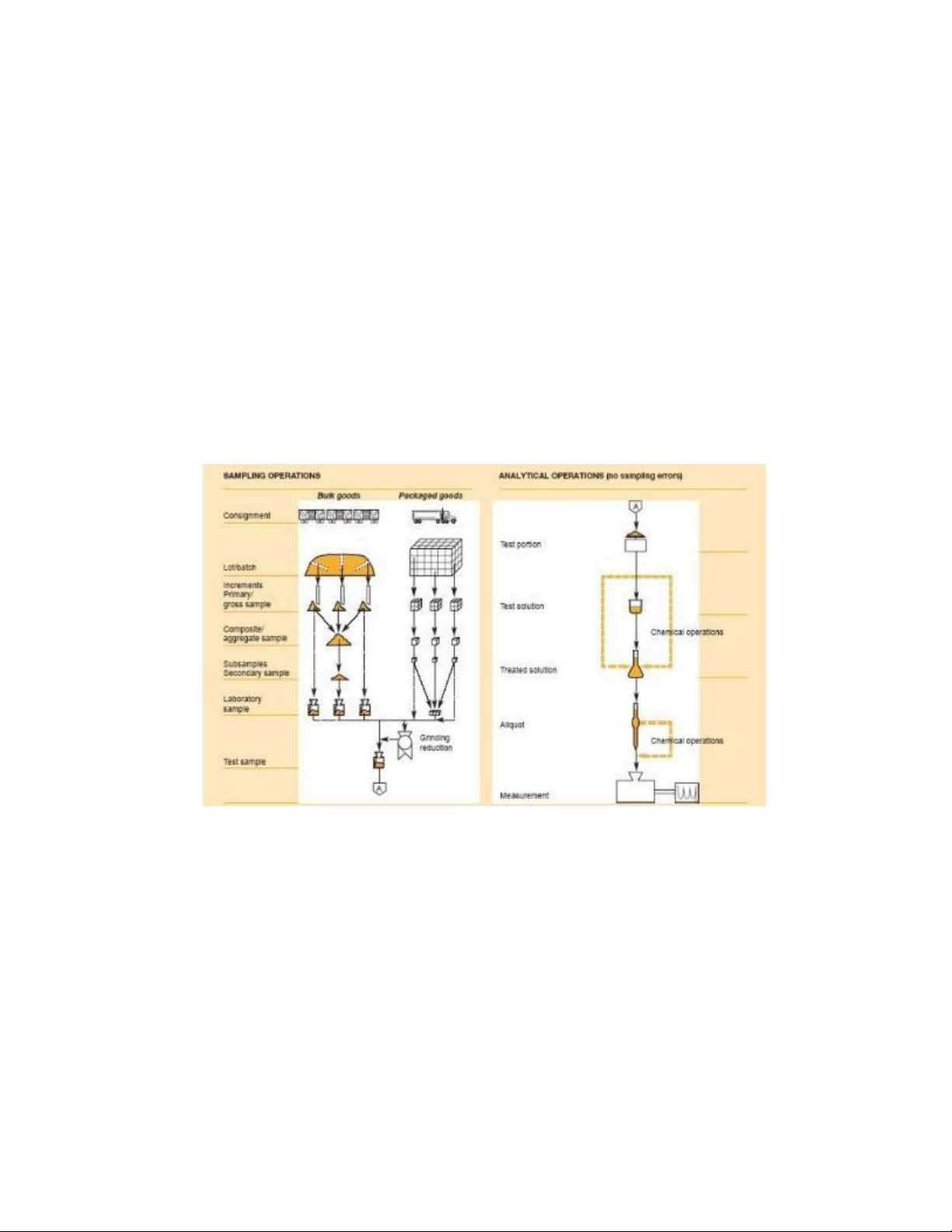
Figure 2.1 illustrates the different stages in sampling and analysis, indicating where sampling errors
may arise as distinct from analytical errors.
− Laboratory sample: sample prepared for sending to the laboratory and intended for inspection or testing.
− Test sample: sample prepared from the laboratory sample according to the procedure specified
in the method of test and from which test portions are taken.
− Test portion: measured (volume or mass) representative sample taken from the laboratory
sample for use in the preparation of the initial suspension.
− Initial suspension: primary dilution suspension, solution or emulsion obtained after a weighed
or measured quantity of the product under examination (or of the test sample prepared from the
product) has been mixed with, normally, a nine-fold quantity of diluent.
Figure 2.1. The relationship of the operation involved in sampling and analysis
3.2. Collecting samples for analysis
3.2.1. Aseptic sampling
Aseptic sampling is a technique used to prevent contamination by the sampling method. Aseptic
sampling involves the use of sterile sampling implements and containers.
There are some methods of material sterilization to be used: lOMoAR cPSD| 58562220 − Dry Heating
+ Red heat: flaming until it glows red (metals: inoculation wires)
+ Flaming: scalpels, spoons, scissors … immerse in ethanol and immerse in ethanol and flame
+ Hot air: glass material (glass flasks, test tubes, pipettes …) in 2 h at 160oC – 170oC − Moist Heating
+ Boiling in water for 5-10 min: vegetative cells are killed, but spores can survive!!
+ Steaming at atmospheric pressure (for sensitive media components)
+ Autoclaving: 15-30 min at 121oC using 1 atm overpressure
− Using pre-sterilized plastic tools
− Ethylene oxide gas: 50oC, RH=30%
− Radiation: plastic bags, Petri dishes
− Ultraviolet radiation: air and surfaces − Filtration: liquid
3.2.2. Preparation of sample for analysis
Homogeneous samples including powders and free flowing liquids and concentrates should be
mixed well before removing a portion for testing (for example, by shaking 25 times). Do not shake
powders immediately before testing as the environment may become contaminated by dust particles.
For heterogeneous products (which contain pieces of different foods), sampling should be carried
out by taking aliquots of each component representative of their proportions in the initial product.
It is also possible to homogenize the whole laboratory sample to allow the sampling of a
homogenized test sample. It may be necessary to mince or to grind the laboratory sample. In this
case, to avoid an excessive rise in temperature, do not mince or grind for more than 1 min.
Products stored frozen should be brought to a consistency that allows sampling (i.e. storing at
ambient temperature for a maximum of 3 h or at 2oC ± 2oC for a maximum of 24 h. Samples should
be tested as soon as possible after that.
Packaged products should be opened aseptically, and if necessary and wearing safety glasses and
gloves (if the package cannot be opened without risk of external contamination), the external




