




Preview text:
VIETNAM NATIONAL UNIVERSITY HO CHI MINH CITY
INTERNATIONAL UNIVERSITY ---o0o--- CHEMICAL ENGINEERING
Carriers: A Report - Laboratory
Experiment 1: MELTING POINT DETERMINATION
Instructor: Dr.Le Quang Phong
Date of submission:18/10/2023 Group 03 :
Student’s full name Student’s ID Nguyễn Tiến Phúc BTCEIU21112 Lê Gia Khương BTCEIU21093 Nguyễn Sỹ Khôi BTCEIU21092 Đinh Nguyễn Quốc Thắng BTCEIU21115 I. Abstract
Melting point determination was a thermal analysis technique to characterize solid crystalline
materials. It is used to identify substances and check their purity. A pure compound would melt
over a narrow temperature range, while impurities would lower and widen the range. The
procedure involved filling a capillary tube with crystals and placing it in a melting point
apparatus that gradually heated the sample and recorded the melting temperature range. There
were multiple factors that influenced the melting point of a pure substance, including how
tightly molecules pack together, molecular size, and electrostatic forces between molecules.
According to thermodynamics, the freezing point of a pure material drops as the amount of an
impurity is increased. The presence of an impurity in a sample will both lower the observed
melting point and cause melting to occur over a “broader range.”
Two materials may or may not have the same structure if their melting points are found to be
identical. The measurement of a melting point is undoubtedly a desirable way to verify a
material’s identity and purity, but it must be combined with measurements from other analytical
techniques in order to unambiguously identify a material and assess the purity In general,
mixtures of distinct materials melt over a temperature range that ends below the melting point
of either of the pure materials; one material functions as an impurity in the other. By mixing
two pure substances with distinct melting points and noting the diminished
“melting point range,” it is possible to identify the differences between them II. Introduction
The melting point is the temperature at which a solid melts and becomes a liquid, it is a useful
physical constant because a pure substance melts at a definite temperature and has a sharp
melting point, while an impure substance has a lower melting point and melts over a wide range
so this method identifies compounds or to check the purity of the compound. For solids, this
method could be easily and accurately determined with only small amounts and can cause the
melting point to spread out over a range of several degrees. The most accurate method was to
record a cooling curve of temperature versus time. If a melting point was sharp, it was a good
indication that the solid was pure. If the melting point was over a wide range, it was most likely that the solid was impure
III. Materials and MethodsMaterials: ●
Melting Point Apparatus and 5 Capillary melting point tubes ● Porcelain mortar with pestle ● Gloves and goggles ●
Urea (pure sample) and Cinnamic acid (pure sample) ● Unknown sample Method:
1. Place the solid organic compounds on a porcelain mortar.
2. Crushing the sample by using porcelain mortar and the pestle until it is powdered.
Then, fill it into melting point tubes.
3. Push the open end of the tube into the compound smoothly. Some of the samples will be at the top of the tube 1
4. Hold the closed end of the capillary tube perpendicular to the table and start shaking
the tube gently on the table surface until the powder moves into the bottom of the tube.
5. Place the capillary melting tubes in the Melting point apparatus chamber. Start with a
setting of two, the temperature should slowly rise. The samples should be observed
continuously so that the melting point of that is not missed. Heat slowly to acquire the
most accurate results. Record the melting range, which begins when the sample first
starts to melt and ends when the sample is completely melted.
6. Identify the unknown in experiment 3 by comparing the melting point that was
recorded from the unknown and compare it with the stats on the substance's data melting point.
IV. Result and Discussion. Result:
Part 1: Determine the melting points of pure substances
In part one of the experiment, we used a melting point meter that housed in the
laboratory to identify two pure substances (urea and cinnamic acid) were melted to determine
their melting point ranges and compared to the known values that each substance melted at.
The correction was obtained and plotted against the observed melting point values. For both
samples, the range would be between 131℃ and 133℃. The results are shown in the data table below
Figure 1:The melting point of Urea 2
Figure 2:The melting point of cinnamic acid
Part 2: Determine the melting points of compounds mixture
In part two of the experiment, the melting point of urea and cinnamic mixtures was
recorded. For the 50:50 mixture, the melting point was at 98.6℃, while the 40:60 mixture had
a higher temperature at 107.5℃. These results indicate that the melting point of the mixtures is
influenced by the relative proportions of the two components.
Figure 3: The melting point of the mixture 50% urea and 50% cinnamic acid
Figure 4: The melting point of the mixture 40% urea and 60% cinnamic acid
Part 3: Identify an unknown substance by melting point
The result we had determined the melting point was 158.1 degrees Celsius, which was
close to Salicylic acid ( Melting point range from 158.5 degrees Celsius to 159.0 degrees
Celsius) and the result was much higher than Adipic acid( Melting point range from 152.0 to
154.0 degrees Celsius). We could conclude that the Unknown substance was Salicylic acid
because the result was closer to them. There were some technical and number errors during the
Laboratory periods, we did not clean the mortar and pestle clearly in the second experiment, so 3
it might have been mixed with the unknown substance in the third experiment, which might be
the reason why the melting point resulted in the experiment 3 was not close to the melting point result range.
Figure 5: The melting point of unknown substance Discussion:
1. Why should samples for melting point determination be finely powdered?
The following are some reasons why samples for melting point analysis should be finely powdered:
● - Uniform melting: Fine powder ensures that the sample warms and melts
evenly throughout. This is crucial for determining a melting point that is precise
since any irregularities in the sample could lead to an imprecise melting point range.
● -Faster heating: Compared to larger chunks or crystals, fine powders heat up
more quickly. This can speed up the process of determining the melting point
and lower the chance that heat breakdown or other undesirable chemical
processes will take place before the melting point is achieved.
● - Accuracy temperature measurement: Because fine powder samples have a
smaller volume and a lower surface area to volume ratio than coarse powder
samples, it is possible to monitor temperatures with greater accuracy, which is
necessary to calculate the melting point accurately.
2. Why is it important that the heating rate be carefully controlled once near the suspected melting point of a sample?
It is important to carefully control the heating rate once near the suspected melting point
of a sample for several reasons:
● - Accurate calculation of the melting point: A controlled heating rate makes this
possible. Overheating can produce inaccurate results, such as a wider melting
point range or the failure to identify tiny phase transitions.
● -Impurity detection: By keeping the heating rate constant, it is simpler to
identify impurities in the sample. Shifts or expansions in the melting point range 4
are frequent signs of impurities. An accurate observation of these changes is
made possible by a controlled rate.
● -Safe handling: Careful heating rate management reduces the risk of
overheating and other potential risks. It enables the operator to respond quickly
and prevent unintentional overheating or sample ignition.
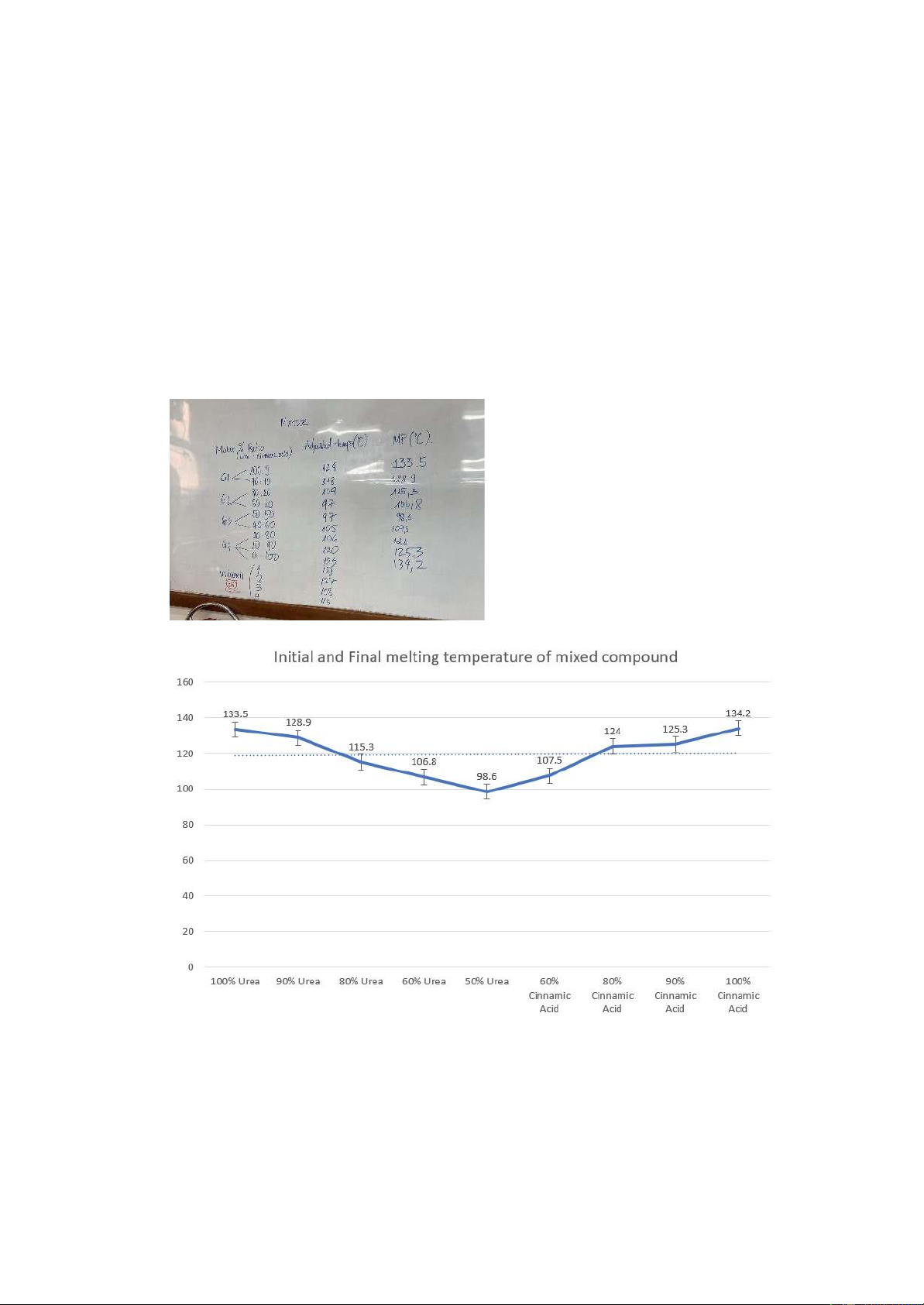
3. Describe and analyze the trend lines for the initial and final melting temperatures versus
composition revealed by the graph you generated in Part 2 of this experiment. To what
extent would it be possible to employ this graph to determine the composition of an
unknown urea and cinnamic acid sample?
The melting temperature of the compound decreases as the mole percentage of urea decreases
until the mole percentages of the two substances are equal and then increases as the mole
percentage of cinnamic acid increases.
We can conclude that the melting temperature of mixed compounds is always lower than that of pure compounds. 5
We can use this graph to determine the composition of an unknown urea and cinnamic acid
sample because when we know the melting temperature of an unknown substance, we can
compare it to this graph. But the melting point of pure Urea and Cinnamic Acid are very close
together, we may add some Urea to the unknown compound and determine the melting
temperature again. If the melting point is unchanged, we can conclude that the unknown
compound is Urea and vice versa. V. Conclusion.
The melting point of numerous different substances and mixtures was recorded from three lab
tests. The pure substances always have a higher melting point than the mixtures. VI. Reference. Lab Manual P. (n.d.). Urea. Urea | NH2CONH2 | CID 1176 - PubChem.
https://pubchem.ncbi.nlm.nih.gov/compound/1176 P.
(n.d.). Cinnamic acid. Cinnamic Acid | C9H8O2 | CID 444539 -
PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/444539 VII. Lab Note.
The melting point is the range of the starting and complete melting temperatures.
The melting point of the mixture is always lower than the melting point of the pure compound.
The melting point of an unknown substance in part 3 is not in the range of Table 1-1. So that
it is not pure compound because the tools weren’t clean.
How do we measure the mass of a mixed compound based on the molar ratio?
For the mixed compound of Urea: Cinnamic Acid (40:60), we can assume that the mole of
urea and cinnamic acid are 0.4 and 0.6, but it is not small enough, so we divide both of them
for 20, so it would be 0.02 and 0.03. Then, we multiply the mole with each Molar mass. (The
same way with 50% Urea: 50% Cinnamic Acid) 6




