





Preview text:
VIETNAM NATIONAL UNIVERSITY HO CHI MINH CITY
INTERNATIONAL UNIVERSITY ---o0o--- CHEMICAL ENGINEERING
Carriers: A Report - Laboratory
Experiment 2: RECRYSTALLIZATION
Instructor: Dr. Le Quang Phong
Date of submission: 25/10/2023 Group 03 Student’s full name Student’s ID Nguyễn Sỹ Khôi BTCEIU21092 Lê Gia Khương BTCEIU21093 Nguyễn Tiến Phúc BTCEIU21112 Đinh Nguyễn Quốc Thắng BTCEIU21115 Table of Contents
I. ABSTRACT ............................................................................................................................................ 3
II. INTRODUCTION ................................................................................................................................ 3
III. MATERIAS AND METHODS ........................................................................................................... 4
1. Materials: ............................................................................................................................................ 4
2. Methods: ............................................................................................................................................. 4
IV. RESULT AND DISCUSSION ............................................................................................................. 5
Result: ...................................................................................................................................................... 5
Discussion: ............................................................................................................................................... 7
V. CONCLUSION ...................................................................................................................................... 7
VI. REFERENCES ..................................................................................................................................... 7
VII. LAB NOTES ........................................................................................................................................ 8 I. ABSTRACT
Recrystallization is a purifying method used in organic chemistry to purify solutes. This method
depends on polarity as the solvents used for recrystallization have a similar polarity to the solvent
to get crystals as a product. After purified one can use percent yield to determine how much of the
chemical was purified and the identity of the solute. In this experiment, an experiment about
recrystallization of crude benzoic acid, and an unknown compound was crystallized and
determined the identity through melting point technique.
The difference between precipitate formation and crystal formation. A precipitate was a solid
mixture of compounds that originated from an oversaturated solution, falling out of it. However,
precipitated substances may not necessarily be pure and could have up to several compounds. On
the one hand, only one compound composed any given crystal. These results in a highly organized,
structurally uniform product; in contrast, precipitate kept no clear structure. Therefore, while
precipitate occurred readily, obtained crystals was a far more complicated process. Although the
procedure of crystallization and recrystallization could display similarities, their respective
definitions diverge. Firstly, crystallization is a separation technique. It involved precipitating
crystals from solution via transitions in the solute’s solubility conditions. The resulting crystals
could be readily distinguished and then filtered out from the solution.
The solubility of most solids increased with elevated temperatures, so recrystallization depended
on the solubility of the solutes in a change of temperatures. The impurities stay in solution while
the desired solid precipitates out. The mechanism of generating crystals involved heating a solution
to a feverish temperature to saturate it. This target elevated temperature often falls near the
solvent’s boiling point. Then, we had to remove the heat source and allow the solution to drop
back to room temperature. As the temperature decreased, so did the solubility of the compound in
solution. Then, the solution became supersaturated. As discussed, these crystals then precipitate
out of the solution at the cooler recrystallization temperature. II. INTRODUCTION
Recrystallization is the process of a crystalline solute dissolved in a hot solvent and then returned
to its solid state when cooled in a solvent by crystalline. Crystal formation was a selective process.
During crystallization, the solute was dissolved in a hot solvent, destroying impurities. Once it had
been heated by the heat plate, then cooled in a cold solvent selectively producing purer crystals.
The size of the crystals figured out how pure the compound was, as larger crystals were more pure
than smaller crystals. The molecules of the impurities did not fit as well causing less pure
compounds to be smaller in size. The main factor in recrystallization was polarity. The solute could
have maximum solubility in the hot solvent and minimum solubility in the cold solvent. The
solvent used to dissolve the compound being used could have a similar polarity. During this
process, either the desired compound or the impurities could exit the solution and leave the solid
compound. Compounds of lower solubility were typically dissolved in methanol, ethanol, and water. III. MATERIAS AND METHODS 1. Materials:
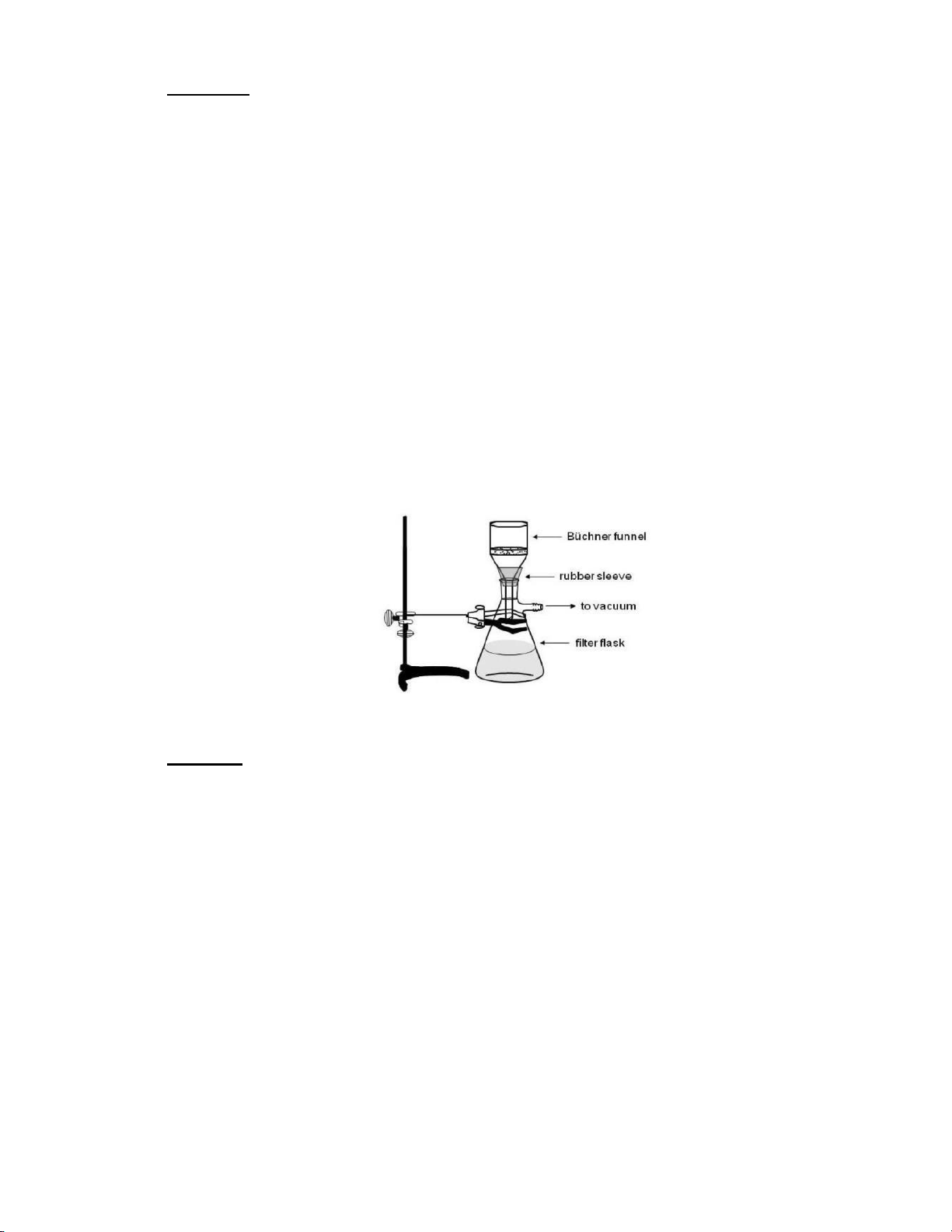
• Vacuum filter and filter paper • 1 Erlenmeyer flask 125 mL • 1 Spatula • 1 Stirring rod • Hot plate • Balance • Aluminum foil • Water bath • 3 50ml beakers • Ice pack
• Methanol – Ethanol - Distilled water • Benzoic acid • Unknown sample
• Melting point apparatus and Capillary melting point tubes
• Porcelain pestle and porcelain mortar
Figure 1. Typical set up for suction, or vacuum filtration. 2. Methods:
• Part 1: Recrystallization of Benzoic
First, weighed 0.5 g of Benzoic acid and placed it into a Beaker. Then placed 20ml of distilled
water into the beaker which has impure Benzoic acid. After that combined stirring the mixture
and boiling it on the hot plate until the mix was absolutely dissolved. When the Benzoic acid
solution is finally invisible, remove the beaker from the hot plate and cover it with aluminum foil
then let the Benzoic acid solution reach room temperature. Place the flask in an ice water bath for
5 minutes to further cool the solution and complete crystallization. After that, collected the crystals
of benzoic acid by vacuum filtration using a Büchner funnel and continued using the spatula to
clean the filter paper to get all the crystals. Finally, dried crystal sample was collected from the
vacuum filtration and weighed for the recovered benzoic acid crystal calculation.
• Part 2: Recrystallization and Identification of Unknown compound
In this part, three beakers with the same unknown compounds from TA and weigh each of them
about 0.5g. In choosing the solvent step, fill each beaker with methanol, ethanol, and water,
respectively. Then continue to observe the results of the samples to see if they are soluble or not.
After that, place the insoluble solvent and continue heating and stirring it on the hot plate until the
mixture is totally dissolved. Get the solution in the water bath and wait for the crystallization and
collect the crystal of the unknown by using Vacuum filtration the same as part 1 and drying the
result. Finally, we used the sample's dried product to crush and determine its melting point to
identify the unknown by comparing it with the material listed in Table 2-1 in the Manual. IV. RESULT AND DISCUSSION Result:
Part 1: Recrystallization of Benzoic acid
In the part one, determined the melting point of recrystallized benzoic acid and the percent %
Recovered = 𝑊𝑒𝑖𝑔ℎ𝑡 𝑜𝑓 𝑏𝑒𝑛𝑧𝑜𝑖𝑐 𝑎𝑐𝑖𝑑 𝑜𝑏𝑡𝑎𝑖𝑛𝑒𝑑 𝑎𝑓𝑡𝑒𝑟 𝑟𝑒𝑐𝑟𝑦𝑠𝑡𝑎𝑊𝑒𝑖𝑔ℎ𝑡 𝑜𝑓 𝑏𝑒𝑛𝑧𝑜𝑖𝑐 𝑎𝑐𝑖𝑑 𝑏𝑒𝑓𝑜𝑟𝑒 𝑟𝑒𝑐𝑟𝑦𝑠𝑡𝑎 𝑙𝑖𝑧𝑎𝑡𝑖𝑜𝑛×100𝑙𝑖𝑧𝑎𝑡𝑖𝑜𝑛
recovered before and after recrystallization of was decided by the formula:
Table 1. The data during performed part 1. Weight of crude Benzoic Acid 0.5g Weight of filter paper 0.27g Actual weight of Benzoic Acid 0.49g Distilled water 20ml
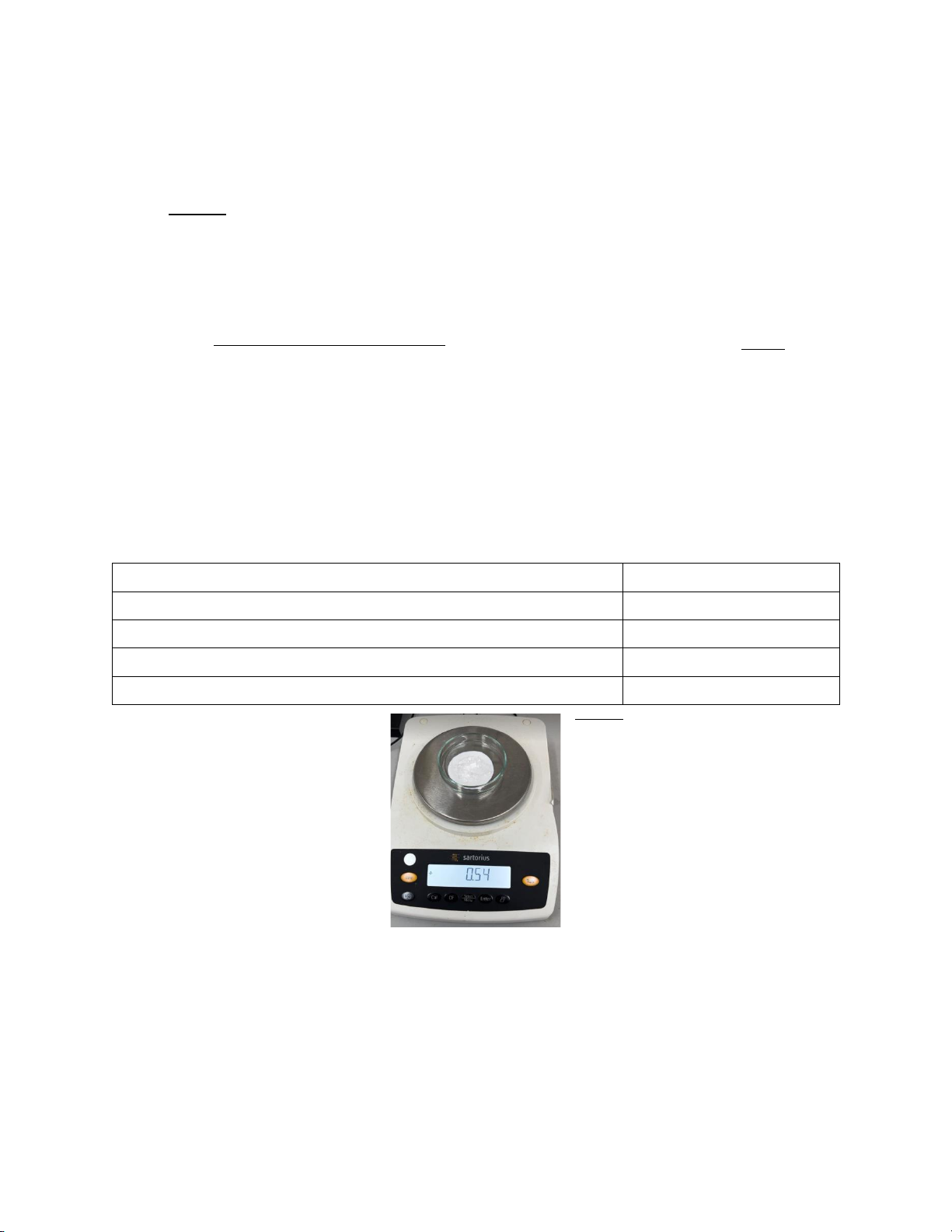
Weight of Benzoic Acid and filter paper after recrystallization 0.54g %Recovered = 0.54−0.270.49×100 =55%
Figure 2. The mass of Benzoic Acid and filter paper after recrystallization.
Part 2: Recrystallization and Identification of Unknown compound
After determined the melting point of the unknown substance is 159.9oC, it could be identified
as Salicylic Acid by comparing it to Table 2-1.
Figure 3. The mass of unknown sample and filter paper after recrystallization.
Table 2-1. Melting point data for the possible unknown samples. Material Melting Point (oC) Benzylic Acid 150-153 Benzoic Acid 122-123 Cholesterol 147-149 2-Nitrobenzoic Acid 140-142 Salicylamide 140-144 Salicylic Acid 158-160 Sulfanilamide 164-166 Trans-Stilbene 122-124
The percent recovered before and after recrystallization of was decided by the formula:
%Recovered = 𝑊𝑒𝑖𝑔ℎ𝑡 𝑜𝑓 𝑢𝑛𝑘𝑛𝑜𝑤𝑛 𝑐𝑜𝑚𝑝𝑜𝑢𝑛𝑑 𝑜𝑏𝑡𝑎𝑖𝑛𝑒𝑑 𝑎𝑓𝑡𝑒𝑟 𝑟𝑒𝑐𝑟𝑦𝑠𝑡𝑎𝑊𝑒𝑖𝑔ℎ𝑡 𝑜𝑓 𝑢𝑛𝑘𝑛𝑜𝑤𝑛 𝑐𝑜𝑚𝑝𝑜𝑢𝑛𝑑 𝑏𝑒𝑓𝑜𝑟𝑒 𝑟𝑒𝑐𝑟𝑦𝑠𝑡𝑎
𝑙𝑖𝑧𝑎𝑡𝑖𝑜𝑛×100 𝑙𝑖𝑧𝑎𝑡𝑖𝑜𝑛
Table 2-2. The data during the performance of part 2.
Weight of crude unknown compound 0.5g Weight of filter paper 0.27g
Actual weight of unknown compound 0.5g Distilled water 20ml
Weight of unknown compound and filter paper after recrystallization 0.57g
%Recovered = 0.57−0.270.5×100 =60%
Figure 4. Melting point result of unknown compound. Discussion:
In part 2, when heating the solvent, it may reach the boiling point so it would reduce the
volume of solvent which mean that it reduces the solubility. In that case, it is necessary to fill more
water (solvent) to increase the volume of solvent back to 20ml so that the solute can be dissolved completely.
In both two parts, it is necessary to wash crystals with the addition of cold distilled water
because it helps remove crystals from the beaker. It must be a cold solvent because it prevents
crystals from dissolving back into the solvent by its low solubility.
A suitable recrystallization solvent must possess key characteristics to efficiently purify a
solid compound. These include high solubility at elevated temperatures, selectivity for dissolving
impurities, non-reactivity with the substances involved, low volatility to minimize solvent loss, a
high boiling point for effective dissolution, low toxicity, affordability, and accessibility, and ease
of removal after recrystallization. The choice of the optimal solvent depends on the specific
properties of the compound and impurities, often requiring experimentation and prior knowledge
for a successful recrystallization process. V. CONCLUSION
In both 2 parts, the crystals recovered were about 55-60%. These results could be increased by
lowering the temperature more to decrease the solubility of the solvent. In both cases, the same
technique was applied so that the crystals collected were purer than the crude ones because of the
recrystallization and filtration. This could be proven by the result that the melting point of the
unknown substance was in the range of the melting point of Salicylic Acid. VI. REFERENCES
• "Organic Chemistry" by Paula Yurkanis Bruice
• "Experimental Organic Chemistry: A Miniscale & Microscale Approach" by John C. Gilbert and Stephen F. Martin VII. LAB NOTES
Figure 5. Lab notes 18/10/2023




