








Preview text:
Table of Contents
I. ABSTRACT ............................................................................................................................................ 3
II. INTRODUCTION ................................................................................................................................ 3
III. MATERIALS AND METHODS ......................................................................................................... 3
• Materials: ............................................................................................................................................. 3
• Methods: ............................................................................................................................................... 4
IV. RESULT AND DISCUSSION .............................................................................................................. 5
• Result: ................................................................................................................................................... 5
• Discussion: ............................................................................................................................................ 9
V. CONCLUSIONS .................................................................................................................................... 9
VI. LAB NOTES ....................................................................................................................................... 10 2 I. ABSTRACT
Fischer esterification is a method used to produce esters and the reaction is reversible,
exhibiting a slow rate of progress. The primary focus was to assess the efficiency of ester synthesis
under varying reaction conditions and investigate the influence of reactant concentrations on
reaction yields. Experimental techniques, including reflux extraction, liquid-liquid extraction, and
simple distillation, were employed to purify and isolate the synthesized ester. In this experiment,
sulfuric acid is used because it is a strong acid and dissociates more completely. After the last step
of the reaction, the organic layer is separated by extracting it with sodium bicarbonate following
the removal of water from the product. As a result, ester is subsequently dehydrated and introduced
into a distillation device for the purpose of purification, as well as to determine its boiling point.
The process entails refluxing the original carboxylic acid with the suitable alcohol in the presence
of an acid catalyst. The acid catalyst donates a proton to the carboxylic acid through the
doublebonded oxygen, resulting in a carbon atom with a deficiency of electrons. The oxygen in
the alcohol functions as a nucleophile, forming a connection with the carbon atom and releasing a
hydrogen atom in the process. As components with varying boiling points are isolated, the
composition of the mixture being distilled alters, hence affecting the temperature necessary for subsequent separation. II. INTRODUCTION
Fischer esterification, also known as the synthesis of ester, is a chemical process in which
an ester is formed by heating a carboxylic acid and an alcohol together in the presence of an acid
catalyst. Alcohol is commonly used as a solvent and, as a result, is found in significant quantities.
Various acids, including sulfuric acid and poisonous acid, can serve as catalysts. In the process of
esterification, a tetrahedral carbonyl addition intermediate is formed, which subsequently
eliminates a water molecule to yield the ester. Ester is one of the most common derivatives of
carboxylic acids and is widely used distributed in both nature and industry. III. MATERIALS AND METHODS 5mL • Materials: Sulfuric o 1 round bottom flask o 1 acid o 16g Cylinder 100 mL Sodium o 1 Cylinder 10 mL o 100mL bicarbonate Butanol o 75mL Acetic acid o o Balance 3
o 1 Beaker 250 mL o 1 Beaker 100 o Distillation apparatus with stand, mL heating mantle and • Methods: water pump
o 1 Separatory funnel 250 mL with stand
o Reflux apparatus with stand, heating mantle and water pump
The methods of Experiment 8 will be divided into 2 separate parts. The first part is the
reflux, weigh and combine 100 mL of n-butanol with 75 mL of acetic acid in a round-bottom flask.
Then, continue adding 5 mL of concentrated H2SO4 into the previous mixture. Put the reflux
device together, using vacuum grease on all the joints. Turn on the water and boil the mixture for
60 minutes while it refluxes. After that, calculate the reaction's theoretical yield. Take the reflux
apart and permit the mixture to cool off. In the second part(extraction and distillation), clamp a
250 mL separatory funnel gently to a ring stand in the hood. Make sure the stopcock is closed.
Carefully transfer the reaction mixture to the funnel, minding the excess amount of acid. After that,
continue using the LLE methods, and repeat the liquid-liquid extraction three times using the
recommended amount of cold water. To be specific, give the funnel a good shake, and give the
layers around ten minutes to separate. Determine which organic layer contains crude butyl acetate
and gather it. Dissolve 16 grams of sodium bicarbonate in 200 mL of water to create a saturated
sodium solution. Use the prepared sodium bicarbonate solution to wash the crude extract many
times in a separatory funnel. Let the layers settle until the aqueous layer has no more visible oil
droplets. Then, while assembling the distillation equipment, take the washed solution and eliminate
the remaining reaction products(during this step, take caution as carbon dioxide pressure builds up
in the funnel). In the next step, to function as the collection flask, preweigh an Erlenmeyer flask
which has been cleaned and dried. At the boiling point of butyl ascetic, collect the distillate. Finally,
weigh the product and determine the ester yield percentage 4 IV. RESULT AND DISCUSSION • Result:
After reflux extraction, the sample was collected and ready for liquid-liquid extraction.
Figure 1: Liquid-liquid extraction process.
The substance was prepared for distillation after impurities got rid of by liquid-liquid extraction. 5
Figure 2: Distillation process.
Table 1: Data sheet 20th Dec 2023. Sample Temperature (oC) Mass (gram) Volume (mL) 1 93 4.33 5 2 113 3.94 5 3 122 4.28 5 4 123 4.33 5 5 122 4.22 5 6 120 3.62 5 7 122 4.48 5 8 123 4.23 5 9 123.5 4.65 5 10 124 4.25 5 11 124.5 4.78 5 12 125 4.28 5 13 124.5 4.34 5 14 124 4.21 5 15 125 4.60 5 16 126 10.53 12
The data gathered indicates that samples' temperatures rise non-uniform, this offers details
regarding the experiment's accuracy. Sample 16 is different from other samples, which was
collected with higher volume (12mL).
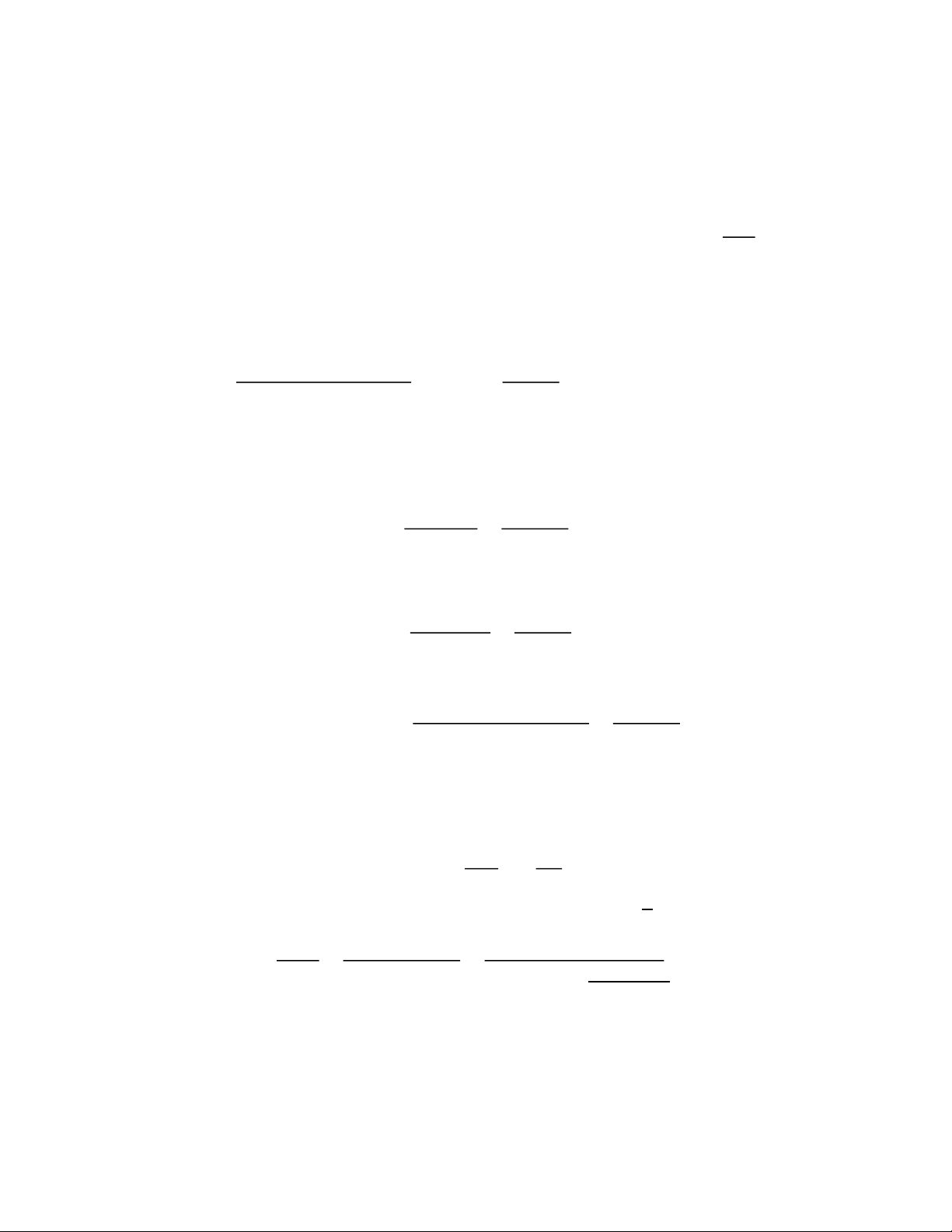
𝑚𝑎𝑐𝑡𝑢𝑎𝑙 𝑦𝑖𝑒𝑙𝑑 𝑒𝑠𝑡𝑒𝑟 = 3.94 + 4.28 + ⋯ + 4.60 + 10.53 = 70.74𝑔
𝑚𝑎𝑐𝑡𝑢𝑎𝑙 𝑦𝑖𝑒𝑙𝑑 𝑒𝑠𝑡𝑒𝑟 70.74𝑔
𝑛𝑎𝑐𝑡𝑢𝑎𝑙 𝑦𝑖𝑒𝑙𝑑 𝑒𝑠𝑡𝑒𝑟 = = 0.61𝑚𝑜𝑙
𝑀𝑊𝐶𝐻3𝐶𝑂𝑂𝐶4𝐻9 116.1583 g _____ 𝑚𝑜𝑙
Theoretical yield of the reation: 6 𝑔
𝑑𝐶𝐻3𝐶𝑂𝑂𝐻 = 1.05 𝑐𝑚3
𝑉𝐶𝐻3𝐶𝑂𝑂𝐻 = 75𝑚𝐿 = 75𝑐𝑚3 𝑔 3 = 78.75𝑔
𝑚𝐶𝐻3𝐶𝑂𝑂𝐻 = 𝑑𝐶𝐻3𝐶𝑂𝑂𝐻 × 𝑉𝐶𝐻3𝐶𝑂𝑂𝐻 = 1.05𝑐𝑚3 × 75𝑐𝑚
𝑛𝐶𝐻3𝐶𝑂𝑂𝐻 = 𝑀𝑊
𝑚𝐶𝐻𝐶𝐻3𝐶𝑂𝑂𝐻3𝐶𝑂𝑂𝐻 = 60.05278.75𝑔𝑔
= 1.31𝑚𝑜𝑙 𝑚𝑜𝑙 𝑔
𝑑𝑛−𝐶4𝐻9𝑂𝐻 = 0.810𝑐𝑚 3
𝑉𝑛−𝐶4𝐻9𝑂𝐻 = 100𝑚𝐿 = 100𝑐𝑚3 𝑔 3 = 81.0𝑔
𝑚𝑛−𝐶4𝐻9𝑂𝐻 = 𝑑𝑛−𝐶4𝐻9𝑂𝐻 × 𝑉𝑛−𝐶4𝐻9𝑂𝐻 = 0.810 𝑐𝑚3 × 100𝑐𝑚
𝑛𝑛−𝐶4𝐻9𝑂𝐻 = 𝑀𝑊
𝑚𝑛−𝐶𝑛−𝐶4𝐻4𝐻9𝑂𝐻9𝑂𝐻 = 74.12381𝑔 𝑔 = 1.1𝑚𝑜𝑙 𝑚𝑜𝑙
Because CH3COOH was excess, the moles of the theoretical yield of the reaction would be the moles of n-C4H9OH 7
𝐶𝐻3𝐶𝑂𝑂𝐻 + 𝐶4𝐻9𝑂𝐻 → 𝐶𝐻3𝐶𝑂𝑂𝐶4𝐻9 + 𝐻2𝑂 1.1 1.1 1.1 (𝑚𝑜𝑙) 𝑔
𝑚𝐶𝐻3𝐶𝑂𝑂𝐶4𝐻9 𝑡ℎ𝑒𝑜𝑟𝑒𝑡𝑖𝑐𝑎𝑙 = 𝑛𝐶𝐻3𝐶𝑂𝑂𝐶4𝐻9 × 𝑀𝑊𝐶𝐻3𝐶𝑂𝑂𝐶4𝐻9 = 1.1𝑚𝑜𝑙 × 116.1583𝑚𝑜𝑙 = 127.8𝑔
Percentage yield of the ester:
𝑚𝑎𝑐𝑡𝑢𝑎𝑙 𝑦𝑖𝑒𝑙𝑑 𝑒𝑠𝑡𝑒𝑟 70.74𝑔 × 100% = × 100% = 55.35%
𝑚𝐶𝐻3𝐶𝑂𝑂𝐶4𝐻9 𝑡ℎ𝑒𝑜𝑟𝑒𝑡𝑖𝑐𝑎𝑙
127.8𝑔 Equilibrium of the reaction: 𝑛𝐶𝐻3𝐶𝑂𝑂𝐻 1.31𝑚𝑜𝑙 [𝐶𝐻3𝐶𝑂𝑂𝐻] = == 17.47𝑀 𝑉𝐶𝐻3𝐶𝑂𝑂𝐻 0.075𝐿 𝑛𝑛−𝐶4𝐻9𝑂𝐻 1.1𝑚𝑜𝑙 [𝐶4𝐻9𝑂𝐻] = == 11𝑀 𝑉𝑛−𝐶4𝐻9𝑂𝐻 0.1𝐿
[𝐶𝐻3𝐶𝑂𝑂𝐶4𝐻9] =
𝑛𝑎𝑐𝑡𝑢𝑎𝑙 𝑦𝑖𝑒𝑙𝑑 𝐶𝐻3𝐶𝑂𝑂𝐶4𝐻9 = 0.61𝑚𝑜𝑙 = 7.92𝑀
𝑉𝑎𝑐𝑡𝑢𝑎𝑙 𝑦𝑖𝑒𝑙𝑑 𝐶𝐻3𝐶𝑂𝑂𝐶4𝐻9 0.077𝐿
𝑛𝑎𝑐𝑡𝑢𝑎𝑙 𝑦𝑖𝑒𝑙𝑑 𝐻2𝑂 = 𝑛𝑎𝑐𝑡𝑢𝑎𝑙 𝑦𝑖𝑒𝑙𝑑 𝐶𝐻3𝐶𝑂𝑂𝐶4𝐻9=0.61𝑚𝑜𝑙 𝑔 𝑔
𝑑𝐻2𝑂 = 1 𝑐𝑚3 = 1 𝑚𝐿 𝑔 𝑚𝐻2𝑂
𝑛𝐻2𝑂 × 𝑀𝑊𝐻2𝑂
0.61𝑚𝑜𝑙 × 18.015𝑚𝑜𝑙 = = 𝑉𝐻2𝑂 = 𝑑𝐻2𝑂 𝑑𝐻2𝑂 1 𝑚𝐿𝑔 = 11𝑚𝐿 𝑛𝐻2𝑂 0.61𝑚𝑜𝑙 8 [𝐻2𝑂] = == 0.06𝑀 𝑉𝐻2𝑂 11𝑚𝐿
[𝐶𝐻3𝐶𝑂𝑂𝐶4𝐻9][𝐻2𝑂] 7.53 × 0.06 𝐾 = == 0.0024
[𝐶𝐻3𝐶𝑂𝑂𝐻][𝐶4𝐻9𝑂𝐻] 17.47 × 11 • Discussion:
Acetic acid is a polar compound; n-butanol is either. So, water is a suitable solvent for the
extraction of acetic acid and n-butanol out of the mixture. They were the remaining substances in
the Fischer Ester Synthesis due to the equilibrium of the reaction. Butyl acetate is lighter than water
so it will be the top layer when performing liquid-liquid extraction. It was easier to collect water
which contained acetic acid and n-butanol out of the mixture. Add more water into the mixture
after collecting aqueous layer. Repeat the process 3 times to maximize the extraction of acetic
acetic and n-butanol. After liquid-liquid extraction, sodium bicarbonate was added to the mixture
to neutralize the sulfuric acid.
Simple distillation technique was performed to take out water from the mixture. The
temperature fluctuated because the distillation of a mixture of compounds can lead to changes in
the composition of the distillation apparatus.
The percentage yield of the ester was 55.35% compared to the theoretical yield of the ester
because the ester synthesis reaction is reversible. It could be increased by adding more acid acetic
or n-butanol in the mixture to shift the reaction to the right and form more ester based on Le
Châtelier’s Principle because the ester synthesis reaction is reversible. In other words, increasing
concentration of reactant(s) will shift the reaction to the right, increase concentration of product(s)
will shift the reaction to the left and vice versa. V. CONCLUSIONS
In conclusion, the Fischer Ester Synthesis experiment concentrated on using sulfuric acid
as a catalyst to catalyze the conversion of butanol and acetic acid into butyl acetate. The successful
synthesis of ester was accomplished by the utilization of many essential processes, such as reflux
extraction, liquid-liquid extraction, and distillation. While liquid-liquid extraction successfully
separated the organic phase containing the intended product, reflux extraction allowed for extended 9
heating, guaranteeing the reaction's completion. The produced butyl acetate was further refined by
distillation. Throughout the experiment, it was clear that sulfuric acid had a catalytic function in
accelerating the process and enabling ester synthesis. To get the best yields, it was emphasized how
important it is to optimize reaction parameters, such as temperature and catalyst concentration.
This extensive practical experience improved competency in basic laboratory procedures and
expanded comprehension of ester synthesis, offering a useful investigation of organic chemistry concepts. VI. LAB NOTES
Figure 3: Lab notes 20th Dec 2023 10




